Static Charges Matter carries electrical charges The charges
![Electrical Conduction in Ionic Ceramics and in Polymers Electrical Conductivity [( -m)-1] Material 105 Electrical Conduction in Ionic Ceramics and in Polymers Electrical Conductivity [( -m)-1] Material 105](https://slidetodoc.com/presentation_image_h2/6eae6111c3975ffbd871f41cfceb0e02/image-15.jpg)

- Slides: 31

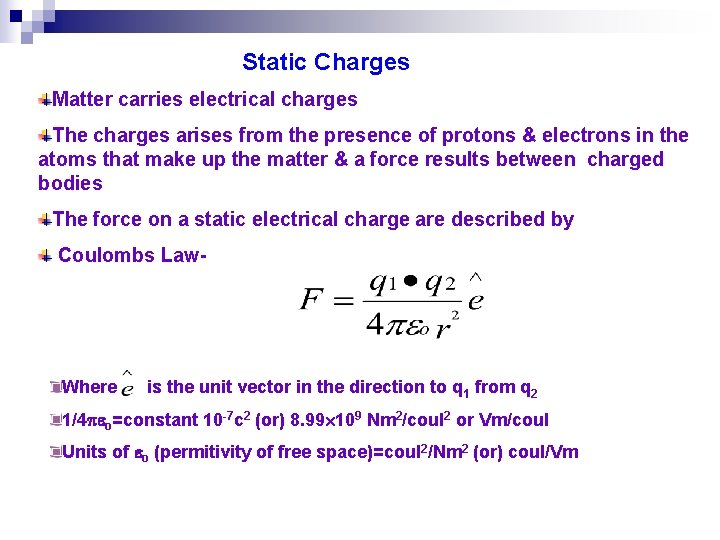
Static Charges Matter carries electrical charges The charges arises from the presence of protons & electrons in the atoms that make up the matter & a force results between charged bodies The force on a static electrical charge are described by Coulombs Law- Where is the unit vector in the direction to q 1 from q 2 1/4 o=constant 10 -7 c 2 (or) 8. 99 109 Nm 2/coul 2 or Vm/coul Units of o (permitivity of free space)=coul 2/Nm 2 (or) coul/Vm

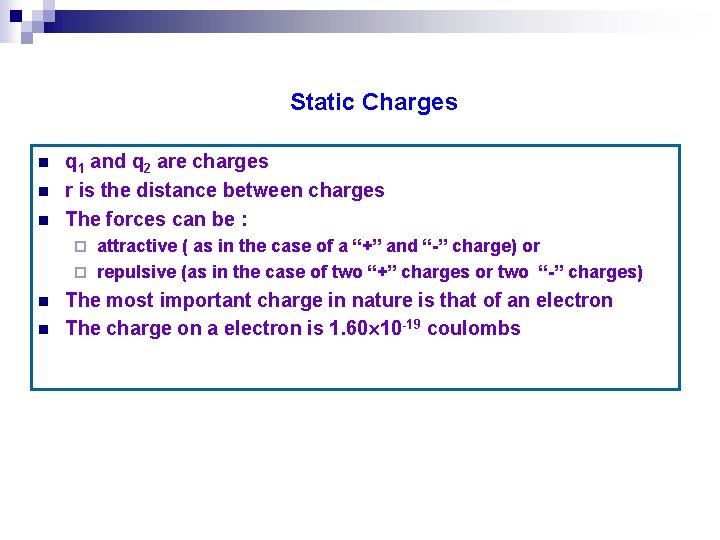
Static Charges n n n q 1 and q 2 are charges r is the distance between charges The forces can be : attractive ( as in the case of a “+” and “-” charge) or ¨ repulsive (as in the case of two “+” charges or two “-” charges) ¨ n n The most important charge in nature is that of an electron The charge on a electron is 1. 60 10 -19 coulombs

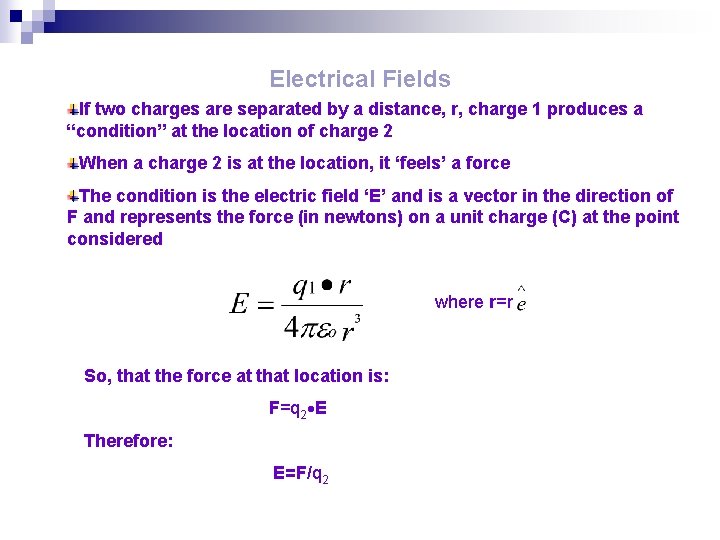
Electrical Fields If two charges are separated by a distance, r, charge 1 produces a “condition” at the location of charge 2 When a charge 2 is at the location, it ‘feels’ a force The condition is the electric field ‘E’ and is a vector in the direction of F and represents the force (in newtons) on a unit charge (C) at the point considered where r=r So, that the force at that location is: F=q 2 E Therefore: E=F/q 2

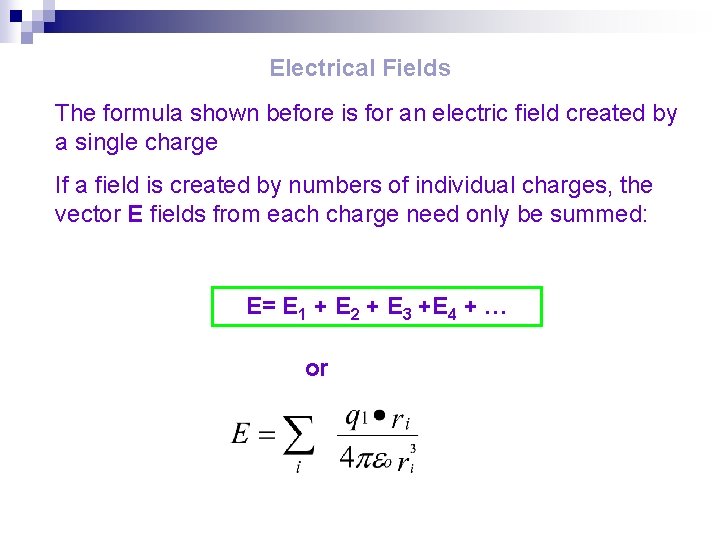
Electrical Fields The formula shown before is for an electric field created by a single charge If a field is created by numbers of individual charges, the vector E fields from each charge need only be summed: E= E 1 + E 2 + E 3 +E 4 + … or

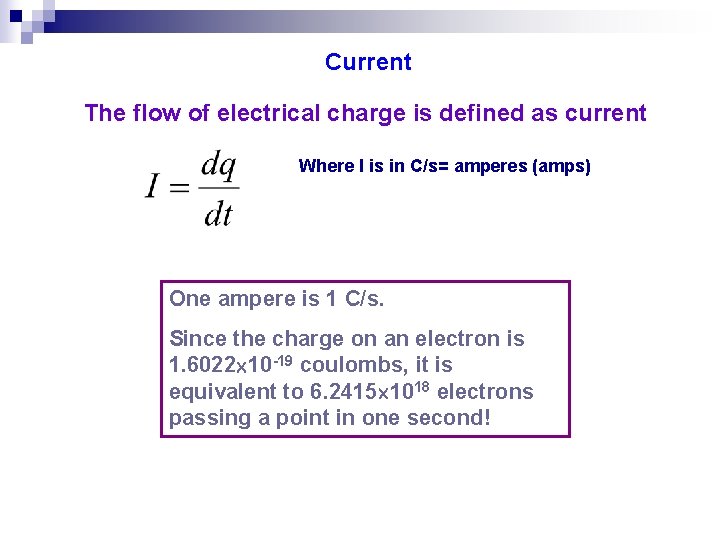
Current The flow of electrical charge is defined as current Where I is in C/s= amperes (amps) One ampere is 1 C/s. Since the charge on an electron is 1. 6022 10 -19 coulombs, it is equivalent to 6. 2415 1018 electrons passing a point in one second!

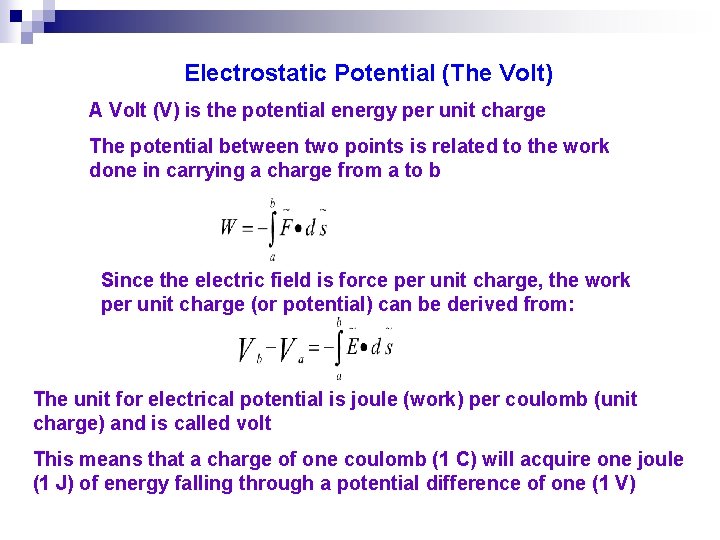
Electrostatic Potential (The Volt) A Volt (V) is the potential energy per unit charge The potential between two points is related to the work done in carrying a charge from a to b Since the electric field is force per unit charge, the work per unit charge (or potential) can be derived from: The unit for electrical potential is joule (work) per coulomb (unit charge) and is called volt This means that a charge of one coulomb (1 C) will acquire one joule (1 J) of energy falling through a potential difference of one (1 V)

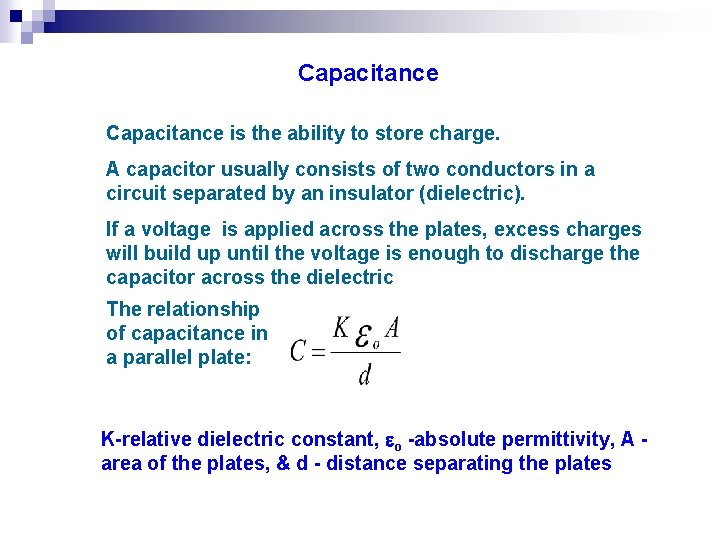
Capacitance is the ability to store charge. A capacitor usually consists of two conductors in a circuit separated by an insulator (dielectric). If a voltage is applied across the plates, excess charges will build up until the voltage is enough to discharge the capacitor across the dielectric The relationship of capacitance in a parallel plate: K-relative dielectric constant, o -absolute permittivity, A area of the plates, & d - distance separating the plates

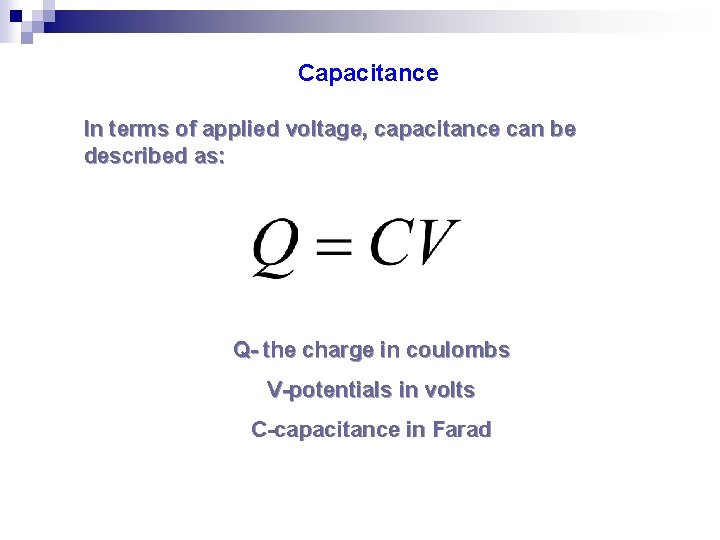
Capacitance In terms of applied voltage, capacitance can be described as: Q- the charge in coulombs V-potentials in volts C-capacitance in Farad

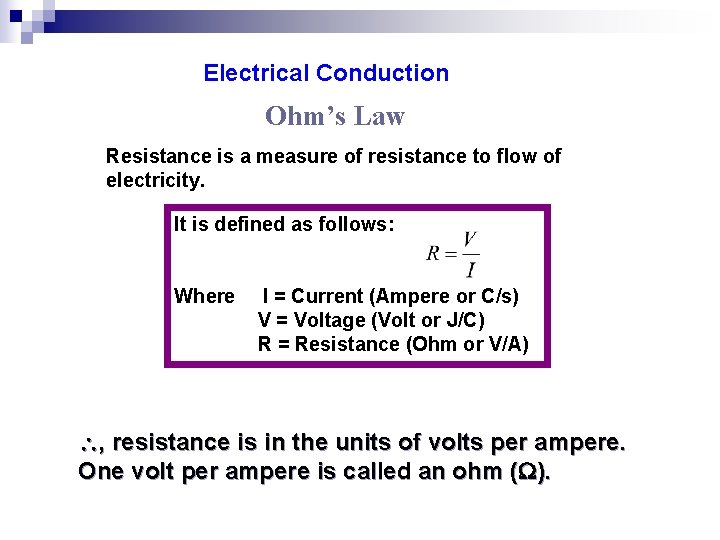
Electrical Conduction Ohm’s Law Resistance is a measure of resistance to flow of electricity. It is defined as follows: Where I = Current (Ampere or C/s) V = Voltage (Volt or J/C) R = Resistance (Ohm or V/A) , resistance is in the units of volts per ampere. One volt per ampere is called an ohm ( ).

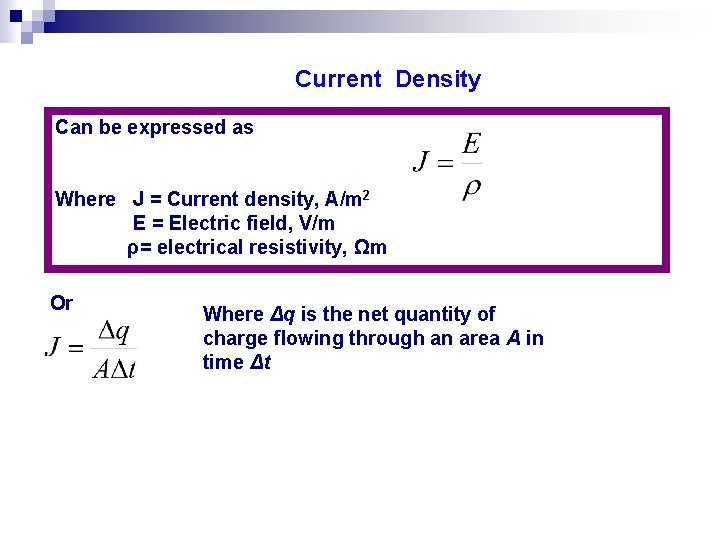
Current Density Can be expressed as Where J = Current density, A/m 2 E = Electric field, V/m ρ= electrical resistivity, Ωm Or Where Δq is the net quantity of charge flowing through an area A in time Δt

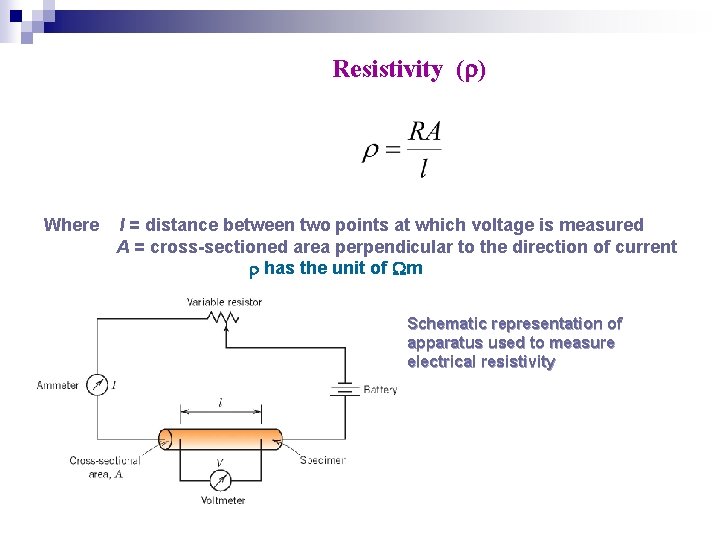
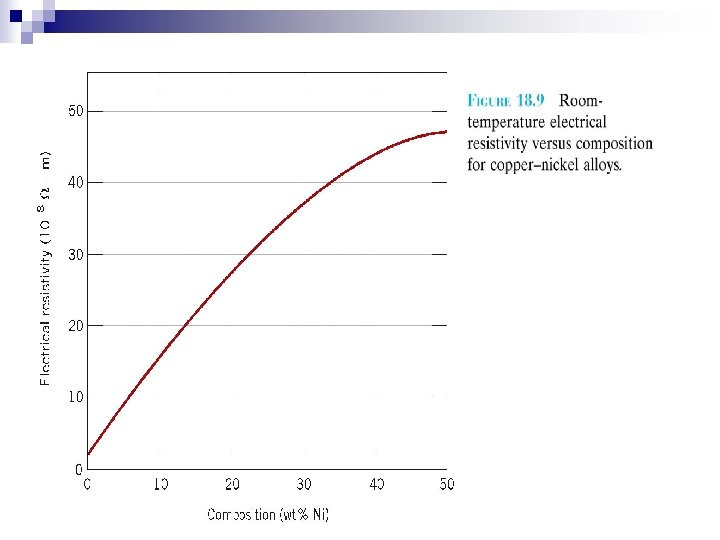
Resistivity ( ) Where l = distance between two points at which voltage is measured A = cross-sectioned area perpendicular to the direction of current has the unit of m Schematic representation of apparatus used to measure electrical resistivity

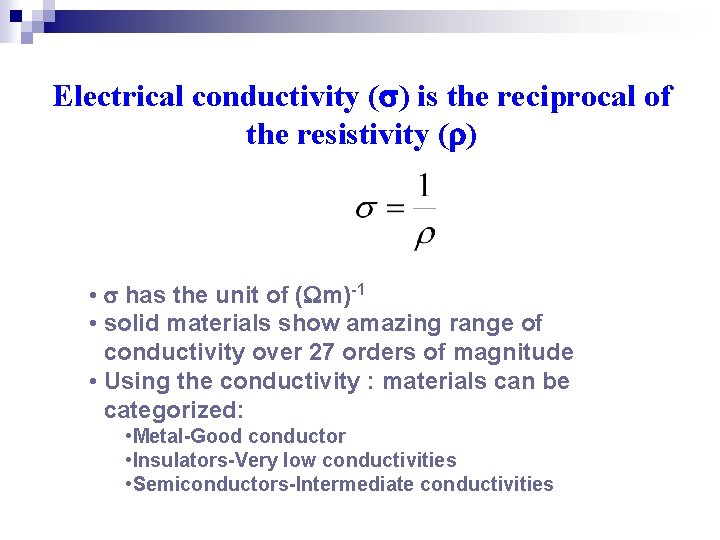
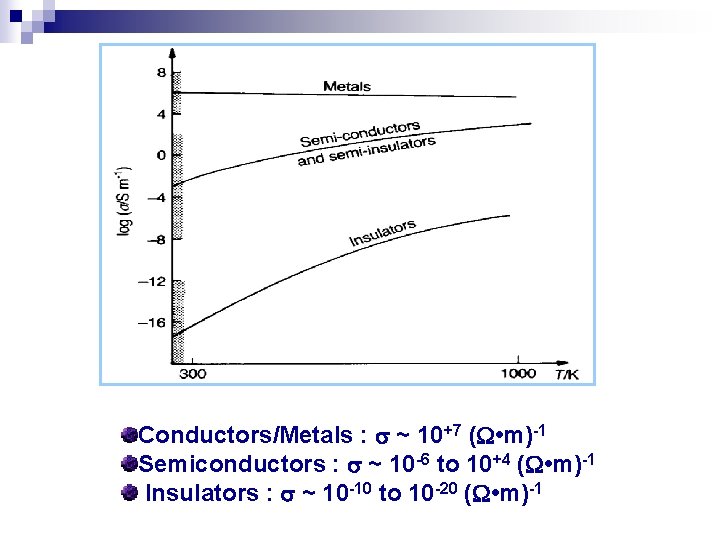
Electrical conductivity ( ) is the reciprocal of the resistivity ( ) • has the unit of ( m)-1 • solid materials show amazing range of conductivity over 27 orders of magnitude • Using the conductivity : materials can be categorized: • Metal-Good conductor • Insulators-Very low conductivities • Semiconductors-Intermediate conductivities

n n n A conductor-a substance that allows current to flow through it In metals, the current is composed of moving electrons Electrolytic solutions also conduct current but by the movement or flow of ions VS §Insulators have few mobile electrons or ions §The flow of current is inhibited §As fields are increased, dielectric breakdown of insulators occurs and the current is discharged as a surge §The dielectric strength is the maximum field an insulator can support

Conductors/Metals : ~ 10+7 ( • m)-1 Semiconductors : ~ 10 -6 to 10+4 ( • m)-1 Insulators : ~ 10 -10 to 10 -20 ( • m)-1
![Electrical Conduction in Ionic Ceramics and in Polymers Electrical Conductivity m1 Material 105 Electrical Conduction in Ionic Ceramics and in Polymers Electrical Conductivity [( -m)-1] Material 105](https://slidetodoc.com/presentation_image_h2/6eae6111c3975ffbd871f41cfceb0e02/image-15.jpg)
Electrical Conduction in Ionic Ceramics and in Polymers Electrical Conductivity [( -m)-1] Material 105 Graphite CERAMICS Aluminum oxide Porcelain Soda-lime glass Mica 10 -10 - 10 -12 < 10 -10 10 -11 - 10 -15 POLYMERS Phenol-formaldehyde Nylon 6, 6 Polymethyl methacrylate Polyethylene Polystyrene Polytetrafluoroethylene 10 -9 - 10 -10 10 -9 - 10 -12 < 10 -12 10 -13 - 10 -17 < 10 -14 < 10 -16 üMost polymers and ionic ceramics are insulators with Eg > 2 e. V üVery small conductivity, then high resistivity ü increases with temperature s

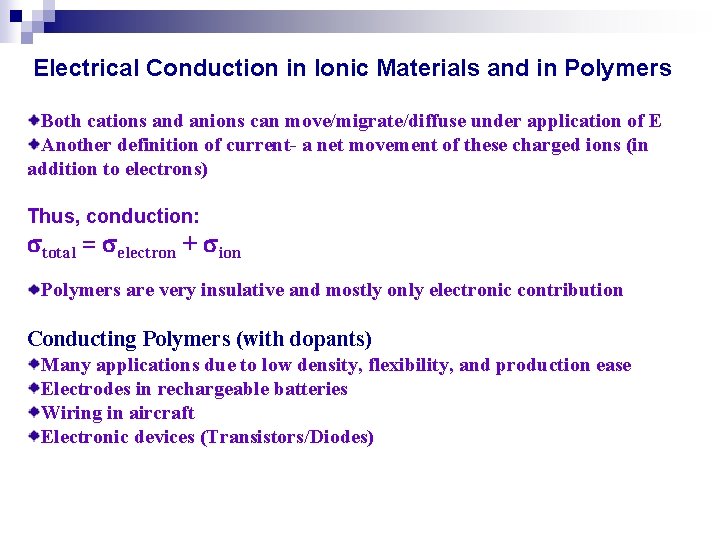
Electrical Conduction in Ionic Materials and in Polymers Both cations and anions can move/migrate/diffuse under application of E Another definition of current- a net movement of these charged ions (in addition to electrons) Thus, conduction: total = electron + ion Polymers are very insulative and mostly only electronic contribution Conducting Polymers (with dopants) Many applications due to low density, flexibility, and production ease Electrodes in rechargeable batteries Wiring in aircraft Electronic devices (Transistors/Diodes)

Electron Conduction in Real Mode Influence of Temperature n Influence of Impurities n Influence of Plastic Deformation n

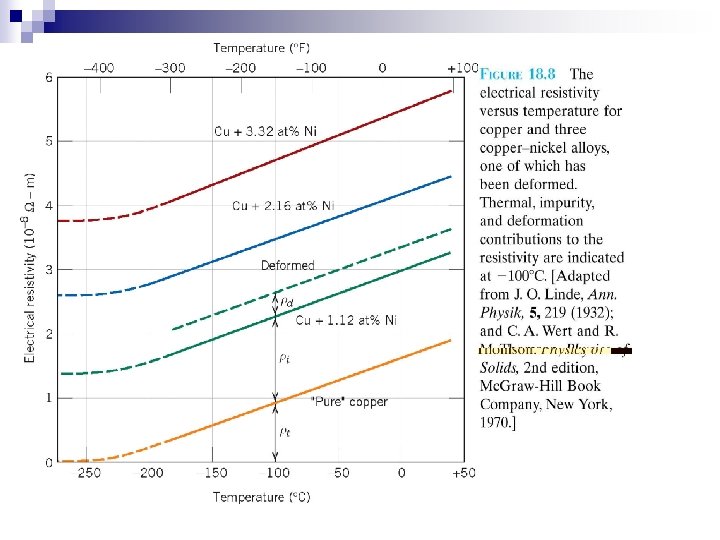
Electrical Resistivity of Metals Resistivity: Scattering of electrons due to crystal defects Increasing the numbers of defect raises resistivity( lowers conductivity) Number of defects depend on temperature, composition, specimen, and thermal history total = t + i + d Matthiessen’s Rule t = thermal contribution i = impurity contribution d = plastic deformation independent

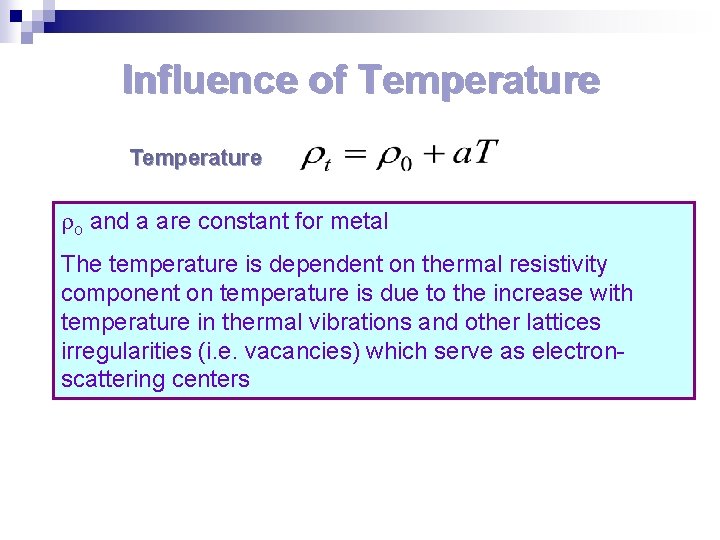
Influence of Temperature o and a are constant for metal The temperature is dependent on thermal resistivity component on temperature is due to the increase with temperature in thermal vibrations and other lattices irregularities (i. e. vacancies) which serve as electronscattering centers


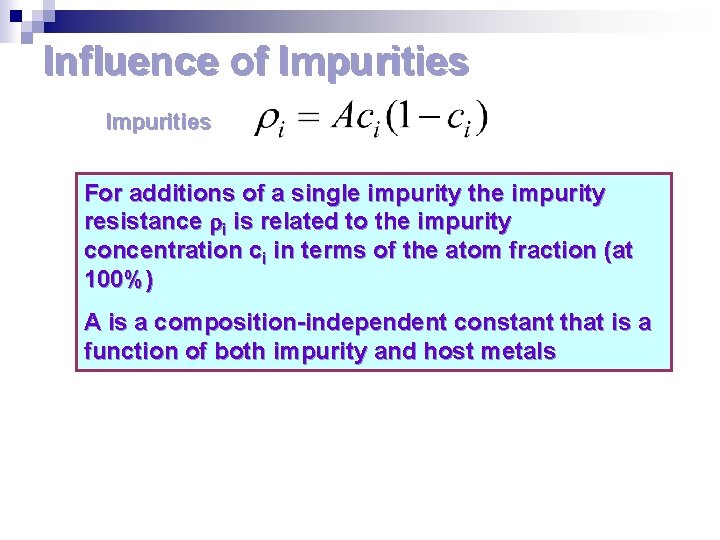
Influence of Impurities For additions of a single impurity the impurity resistance i is related to the impurity concentration ci in terms of the atom fraction (at 100%) A is a composition-independent constant that is a function of both impurity and host metals


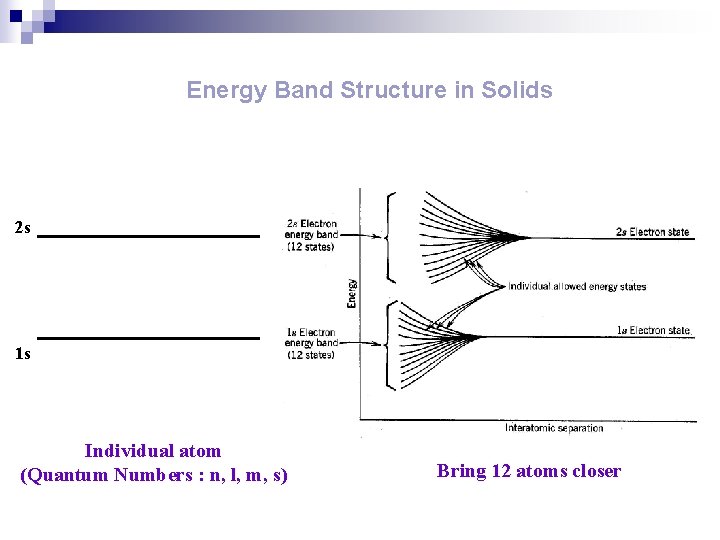
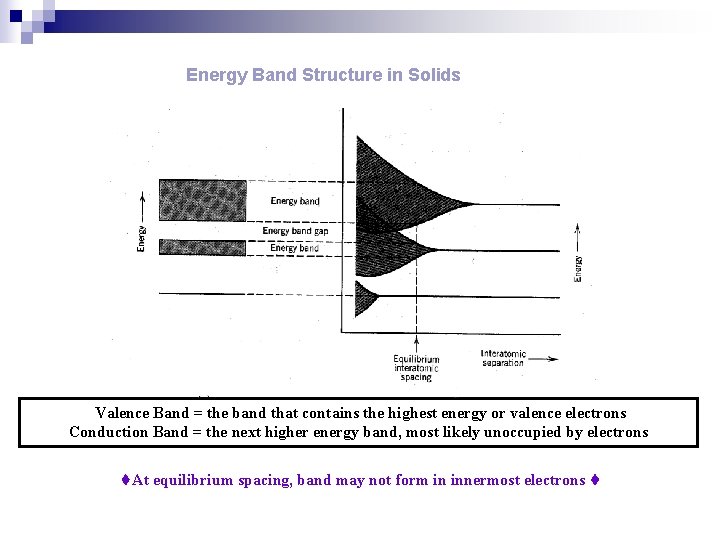
ENERGY BAND n. A range of energies that electrons can have in a solid. In a single atom, electrons can exist in discrete energy levels. n. In a crystal, in which large numbers of atoms are held closely together in a lattice, electrons are influenced by a number of adjacent nuclei and the sharply defined levels of the atoms become bands of allowed energy; this approach to energy levels in solids is often known as the band theory. n. Each band represents a large number of allowed quantum states. n. Between the bands are forbidden bands. n. The outermost electrons of the atoms form the valence band. This is the band, of those occupied, that has the highest energy.

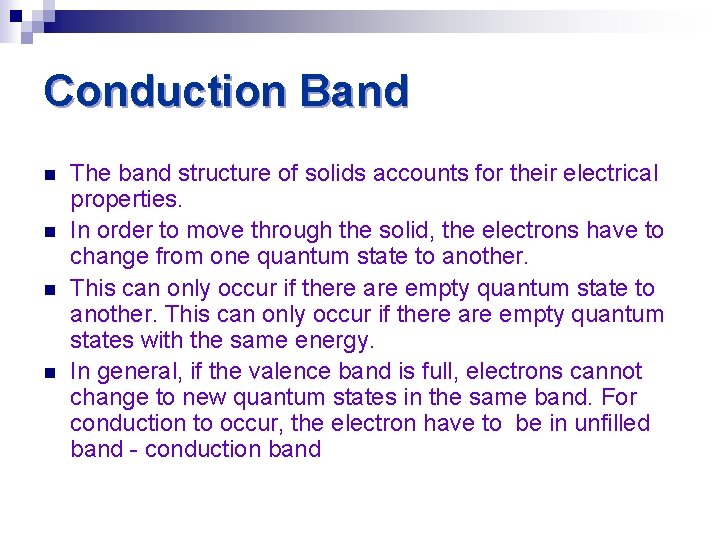
Conduction Band n n The band structure of solids accounts for their electrical properties. In order to move through the solid, the electrons have to change from one quantum state to another. This can only occur if there are empty quantum states with the same energy. In general, if the valence band is full, electrons cannot change to new quantum states in the same band. For conduction to occur, the electron have to be in unfilled band - conduction band

Energy Band Structure in Solids 2 s 1 s Individual atom (Quantum Numbers : n, l, m, s) Bring 12 atoms closer

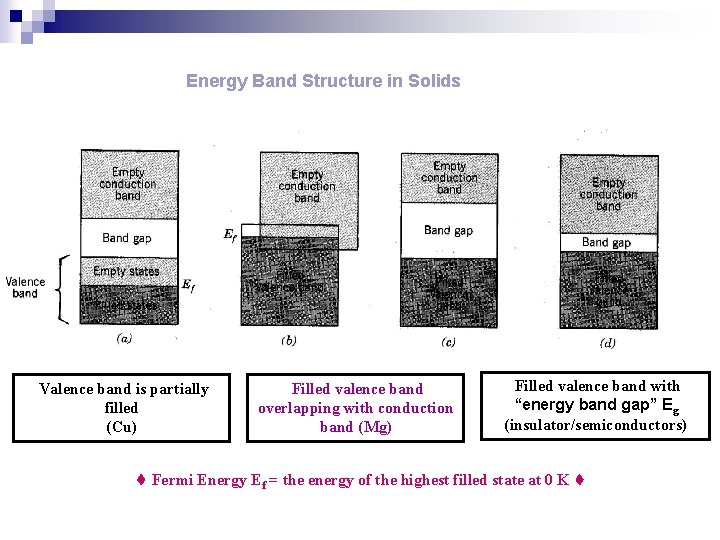
Energy Band Structure in Solids Valence Band = the band that contains the highest energy or valence electrons Conduction Band = the next higher energy band, most likely unoccupied by electrons At equilibrium spacing, band may not form in innermost electrons

Energy Band Structure in Solids Valence band is partially filled (Cu) Filled valence band overlapping with conduction band (Mg) Filled valence band with “energy band gap” Eg (insulator/semiconductors) Fermi Energy Ef = the energy of the highest filled state at 0 K

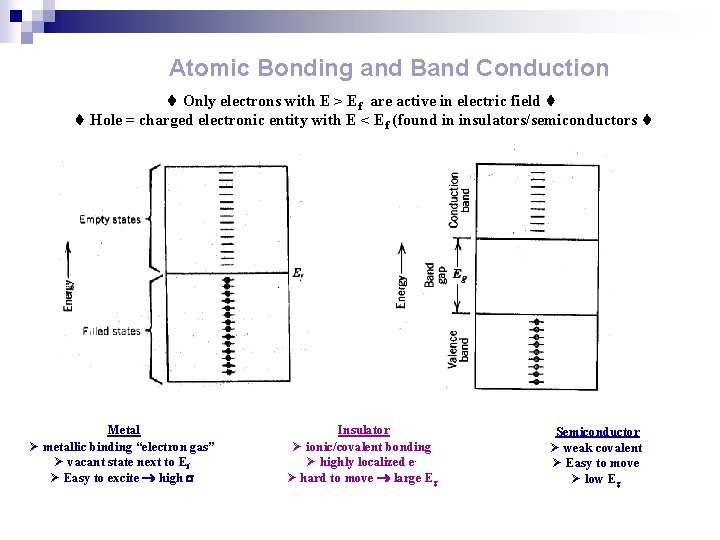
Atomic Bonding and Band Conduction Only electrons with E > Ef are active in electric field Hole = charged electronic entity with E < Ef (found in insulators/semiconductors Metal Ø metallic binding “electron gas” Ø vacant state next to Ef Ø Easy to excite high Insulator Ø ionic/covalent bonding Ø highly localized eØ hard to move large Eg Semiconductor Ø weak covalent Ø Easy to move Ø low Eg

Ferroelectricity • Exhibit spontaneous polarization which is polarization in the absence of electric field • Materials exhibit ferroelectric properties: Barium Titanate, Rochelle salt, potassium dihydrogen phosphate, potassium niobate, lead zirconate-titanate • Have extreme high dielectric constant at relatively low applied field frequencies • Capacitors from this material much smaller than other dielectric materials

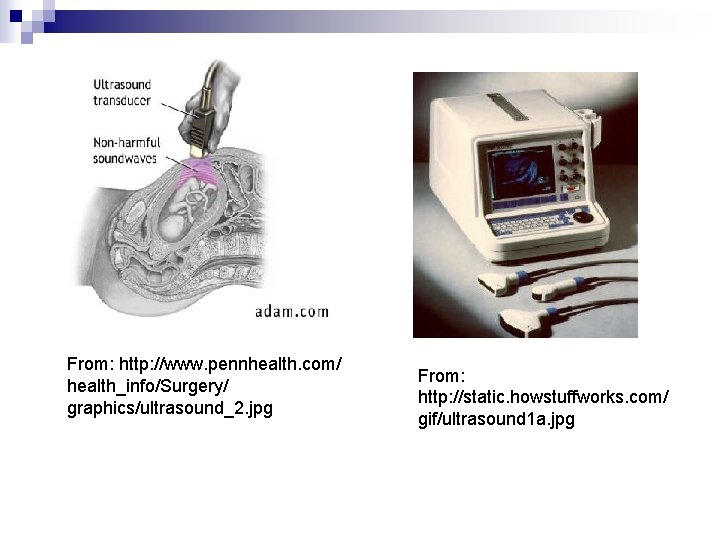
Piezoelectricity • Pressure electricity • Polarization is induced and electric field is established across a specimen by the application of external forces • Utilized in transducers. For instance, some transducer will convert electrical energy into mechanical strains or vice versa • Piezoelectric materials: titanates of barium & lead, lead zirconate, ammonium dihydrogen phosphate & quartz • A common application of piezoelectric material ultrasound transducer

From: http: //www. pennhealth. com/ health_info/Surgery/ graphics/ultrasound_2. jpg From: http: //static. howstuffworks. com/ gif/ultrasound 1 a. jpg