Sponge Set up Cornell Notes on pg 23








- Slides: 41

Sponge: Set up Cornell Notes on pg. 23 Topic: 8. 2 Cellular Respiration: Oxidative Phosphorylation Essential Question: Compare oxidative phosphorylation to photosystems (II/I) in photosynthesis. 8. 2 Cellular Respiration: Oxidative Phosphorylation Compare oxidative phosphorylation to photosystems (II/I) in photosynthesis. Key Vocabulary: Oxidative Phosphorylation • Get papers from tray BIOZONE: 84, 313 Text: 92 -93, 98 -99 Digital Text: 140 -141 Upcoming Due Dates: Biozones due Thurs Test + Notebook check Fri Lab Report due Mon 9/17

Objective SWU: the transfer of electrons through the ETC drives ATP synthase SW: annotate a diagram of oxidative phosphorylation

Understandings: • Phosphorylation of molecules makes them less stable

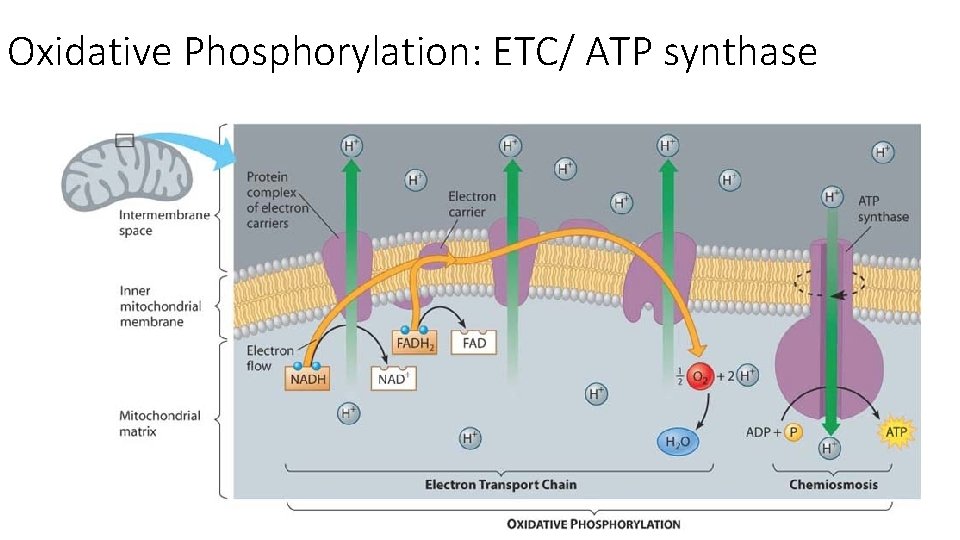
Oxidative Phosphorylation: ETC/ ATP synthase

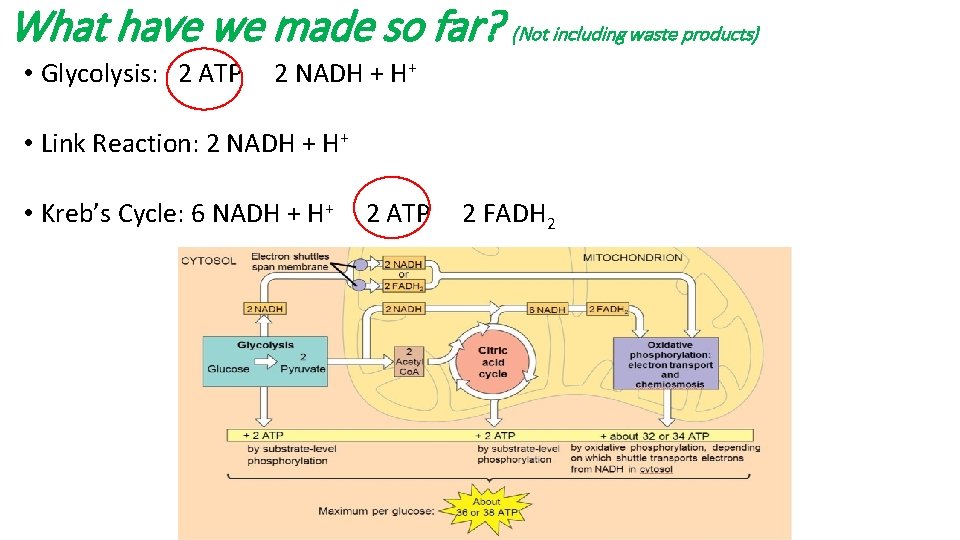
What have we made so far? (Not including waste products) • Glycolysis: 2 ATP 2 NADH + H+ • Link Reaction: 2 NADH + H+ • Kreb’s Cycle: 6 NADH + H+ 2 ATP 2 FADH 2

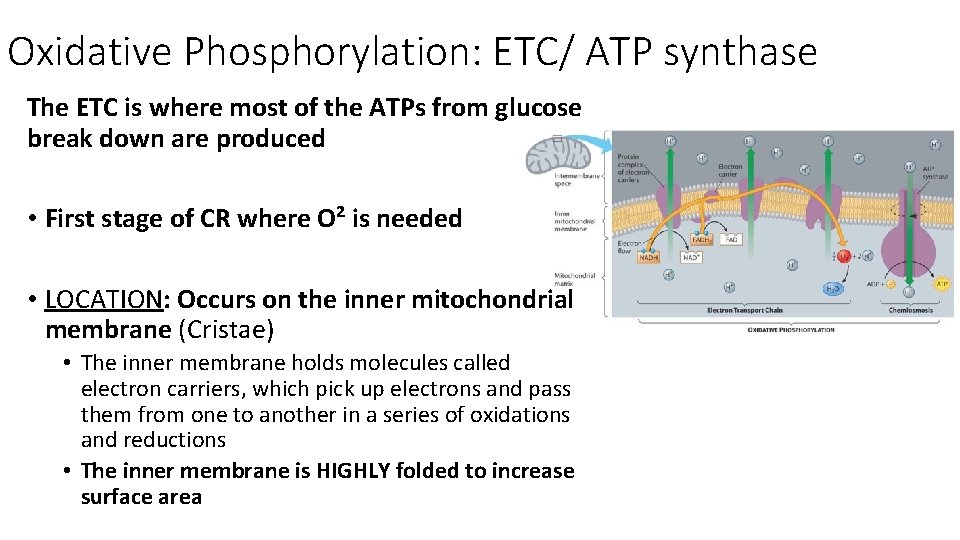
Oxidative Phosphorylation: ETC/ ATP synthase The ETC is where most of the ATPs from glucose break down are produced • First stage of CR where O² is needed • LOCATION: Occurs on the inner mitochondrial membrane (Cristae) • The inner membrane holds molecules called electron carriers, which pick up electrons and pass them from one to another in a series of oxidations and reductions • The inner membrane is HIGHLY folded to increase surface area

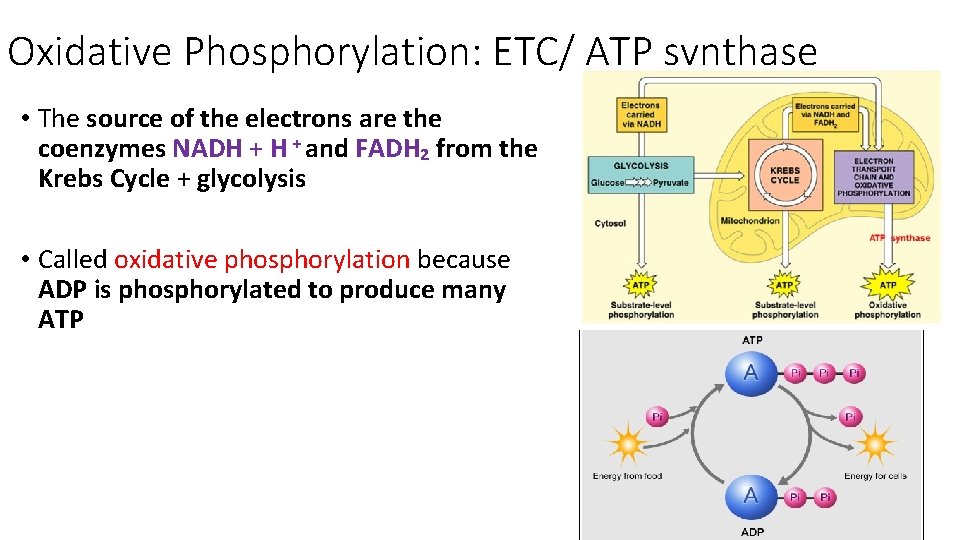
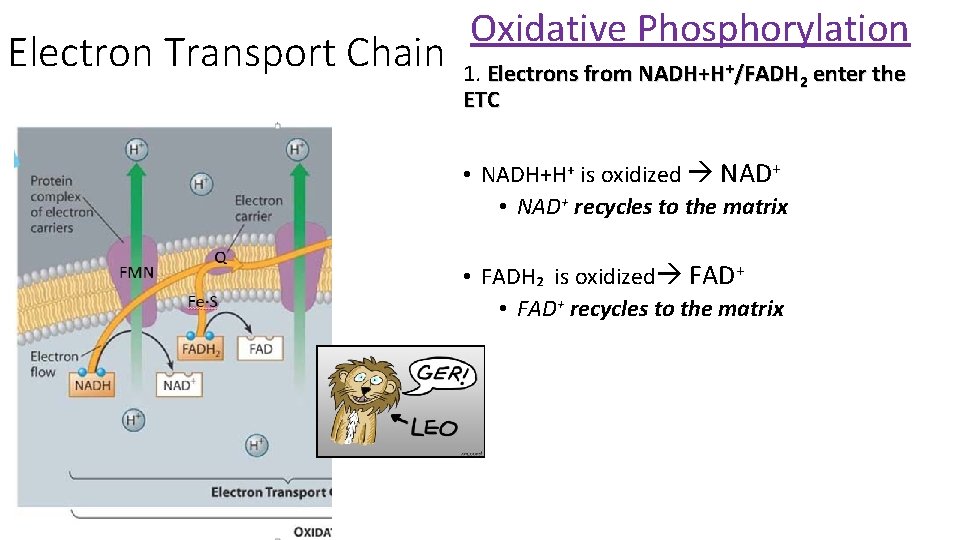
Oxidative Phosphorylation: ETC/ ATP synthase • The source of the electrons are the coenzymes NADH + and FADH₂ from the Krebs Cycle + glycolysis • Called oxidative phosphorylation because ADP is phosphorylated to produce many ATP

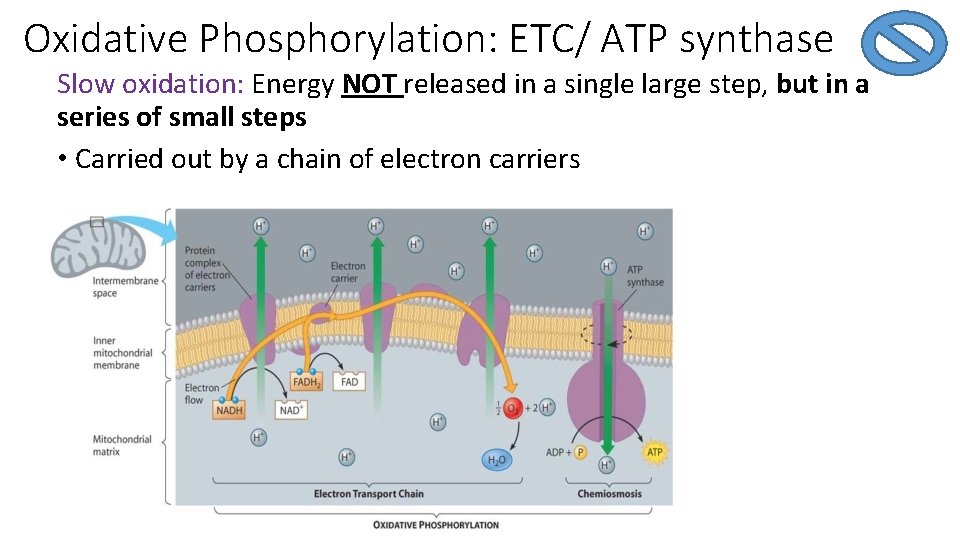
Oxidative Phosphorylation: ETC/ ATP synthase Slow oxidation: Energy NOT released in a single large step, but in a series of small steps • Carried out by a chain of electron carriers

ETC Comparison • Go back to p. 8 in notebook. LDR preview • With your table, compare & contrast the ETC in the LDR with the ETC in oxidative phosphorylation (verbally)

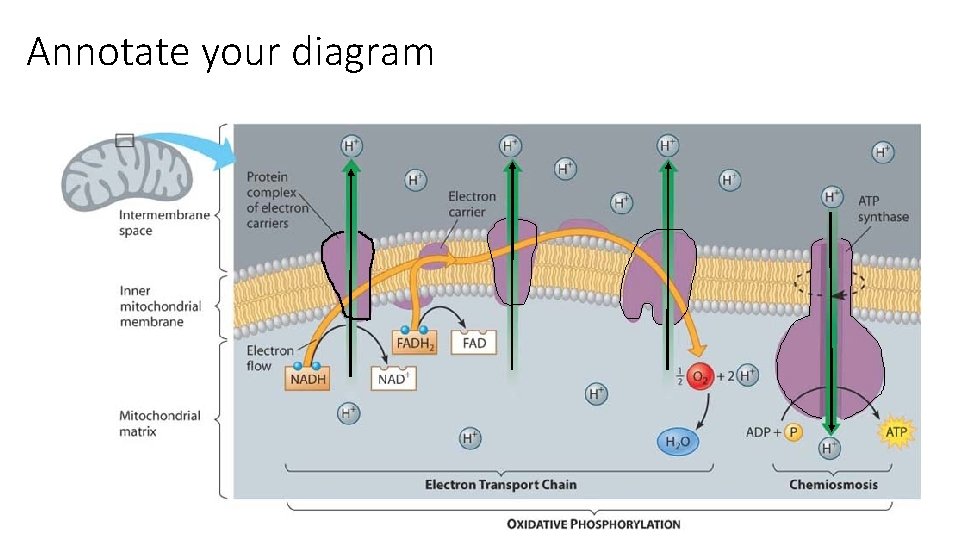
Annotate your diagram

Electron Transport Chain Oxidative Phosphorylation 1. Electrons from NADH+H+/FADH 2 enter the ETC • NADH+H+ is oxidized NAD+ • NAD+ recycles to the matrix • FADH₂ is oxidized FAD+ • FAD+ recycles to the matrix

Understandings: • Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping

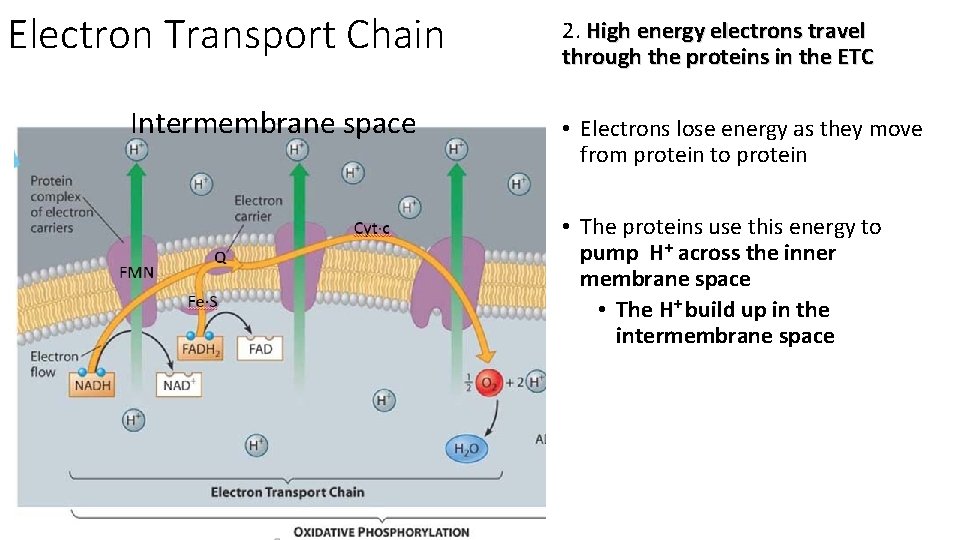
Electron Transport Chain Intermembrane space 2. High energy electrons travel through the proteins in the ETC • Electrons lose energy as they move from protein to protein • The proteins use this energy to pump H+ across the inner membrane space • The H+ build up in the intermembrane space

Understandings: • Oxygen is needed to bind with the free protons to maintain the hydrogen gradient, resulting in the formation of water

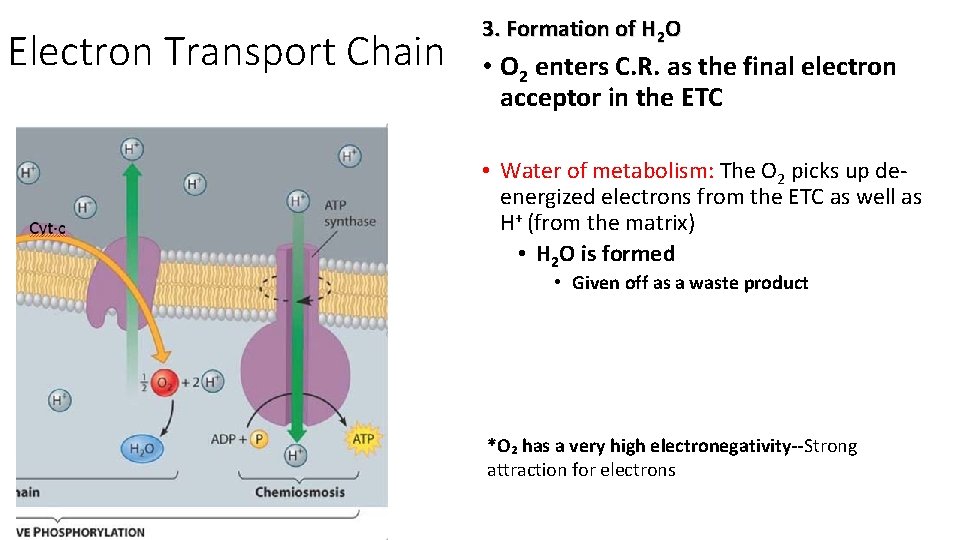
Electron Transport Chain 3. Formation of H 2 O • O 2 enters C. R. as the final electron acceptor in the ETC • Water of metabolism: The O 2 picks up deenergized electrons from the ETC as well as H+ (from the matrix) • H 2 O is formed • Given off as a waste product *O₂ has a very high electronegativity--Strong attraction for electrons

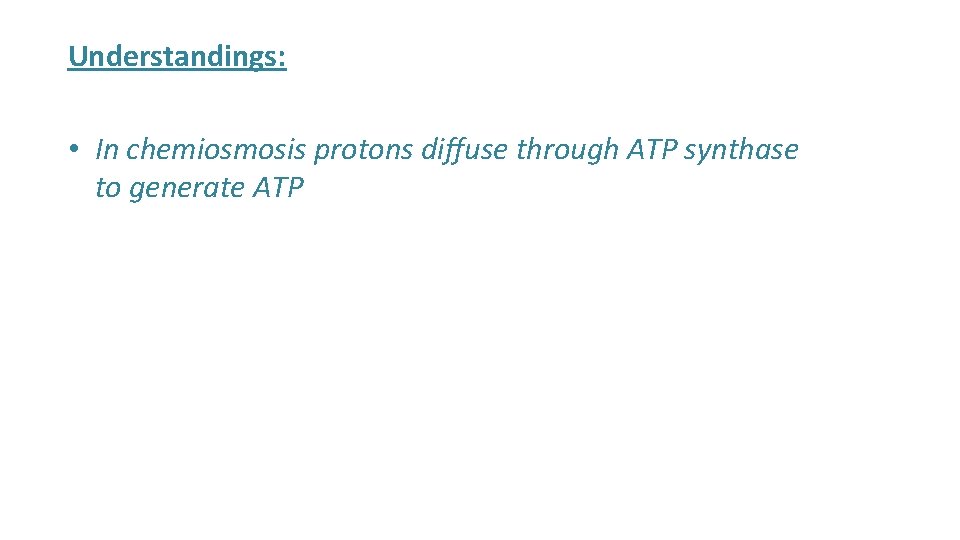
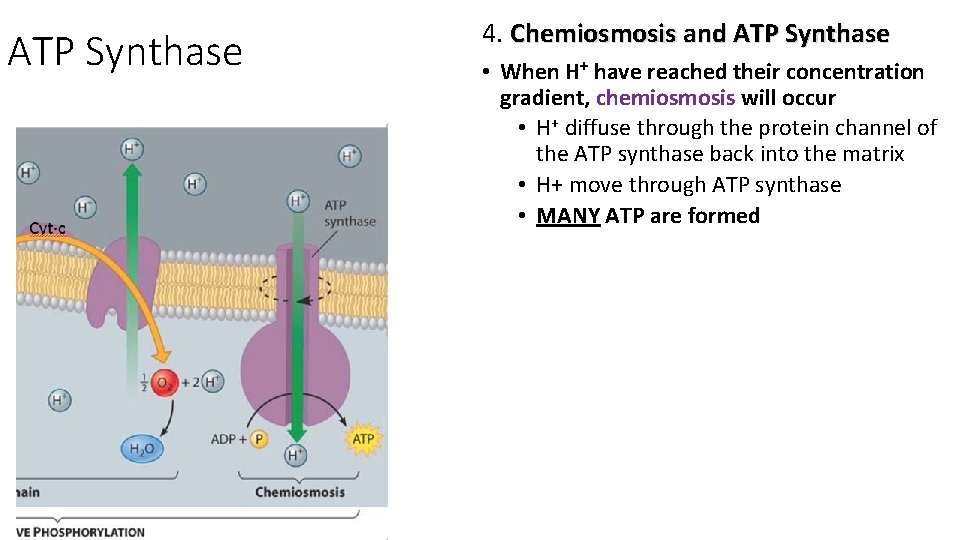
Understandings: • In chemiosmosis protons diffuse through ATP synthase to generate ATP

ATP Synthase 4. Chemiosmosis and ATP Synthase • When H+ have reached their concentration gradient, chemiosmosis will occur • H+ diffuse through the protein channel of the ATP synthase back into the matrix • H+ move through ATP synthase • MANY ATP are formed

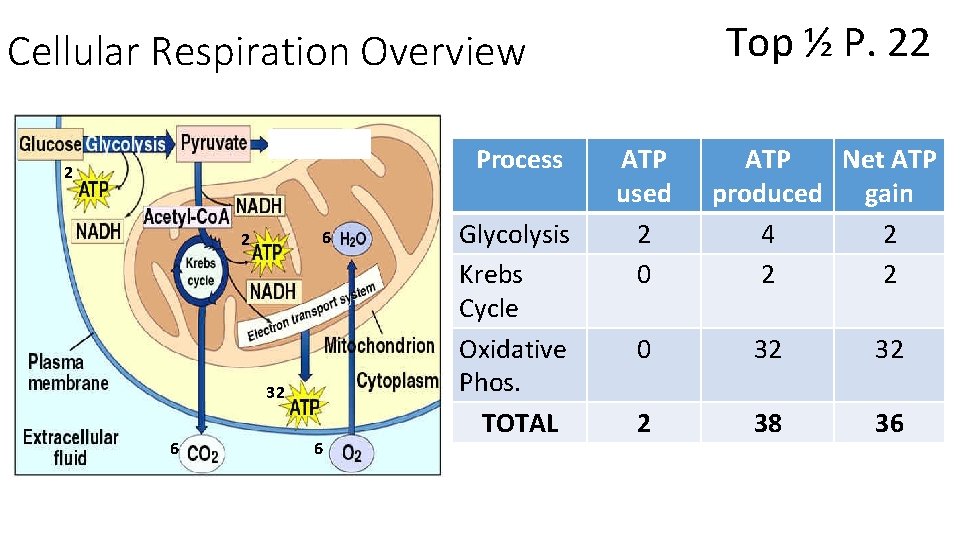
P. 22 Cellular Respiration Overview Table Respiration Worked Example (in Class)

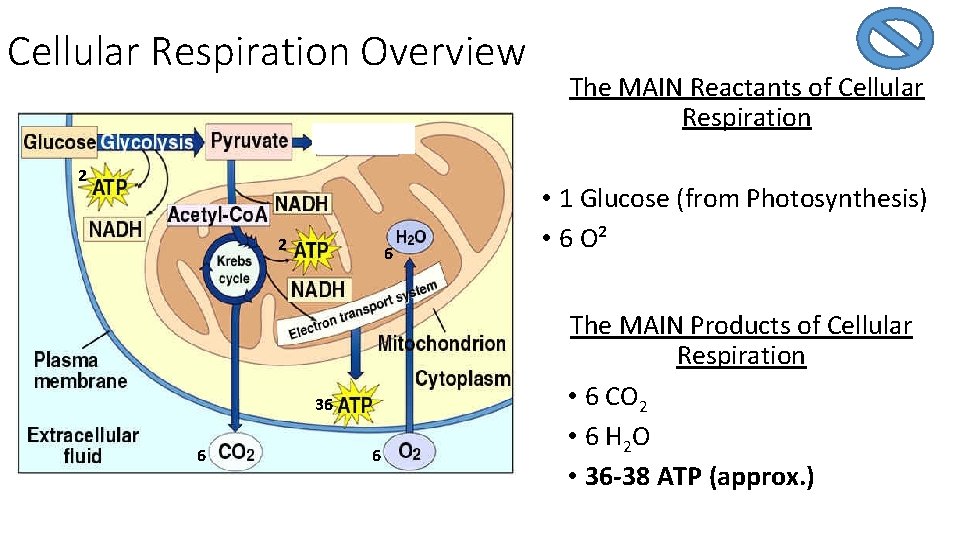
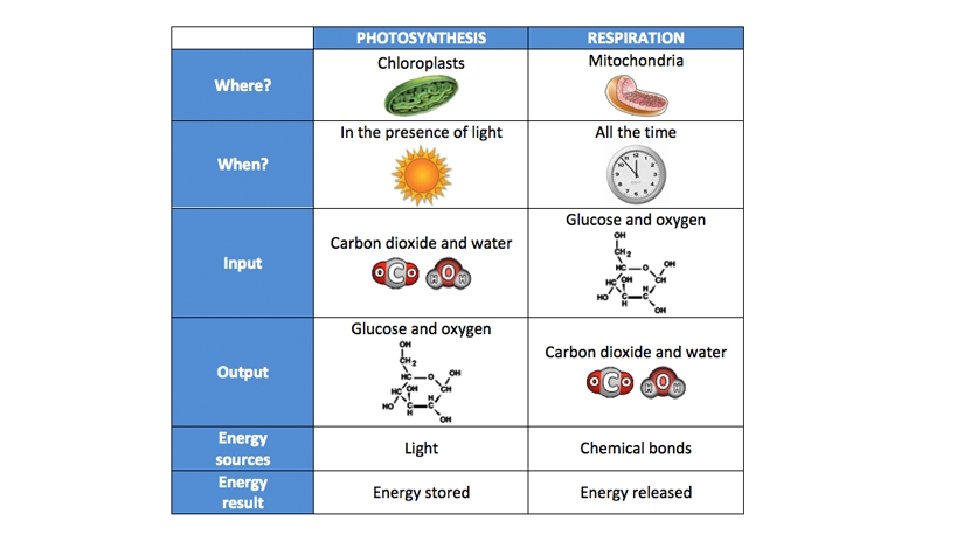
Cellular Respiration Overview 2 2 6 36 6 6 The MAIN Reactants of Cellular Respiration • 1 Glucose (from Photosynthesis) • 6 O² The MAIN Products of Cellular Respiration • 6 CO 2 • 6 H 2 O • 36 -38 ATP (approx. )

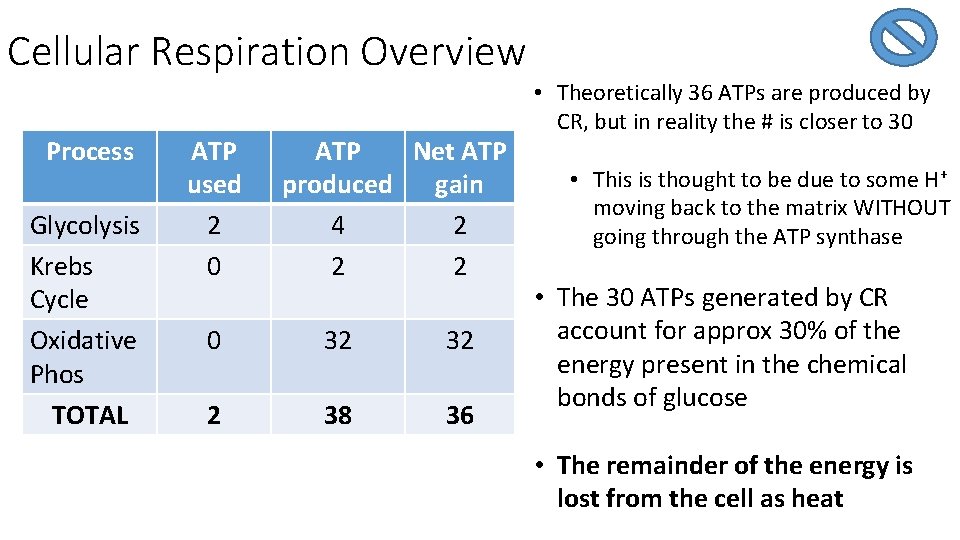
Top ½ P. 22 Cellular Respiration Overview Process 2 6 2 32 6 6 Glycolysis Krebs Cycle Oxidative Phos. TOTAL ATP used 2 0 ATP Net ATP produced gain 4 2 2 2 0 32 32 2 38 36

Cellular Respiration Overview Process Glycolysis Krebs Cycle Oxidative Phos TOTAL ATP used 2 0 ATP Net ATP produced gain 4 2 2 2 0 32 32 2 38 36 • Theoretically 36 ATPs are produced by CR, but in reality the # is closer to 30 • This is thought to be due to some H+ moving back to the matrix WITHOUT going through the ATP synthase • The 30 ATPs generated by CR account for approx 30% of the energy present in the chemical bonds of glucose • The remainder of the energy is lost from the cell as heat

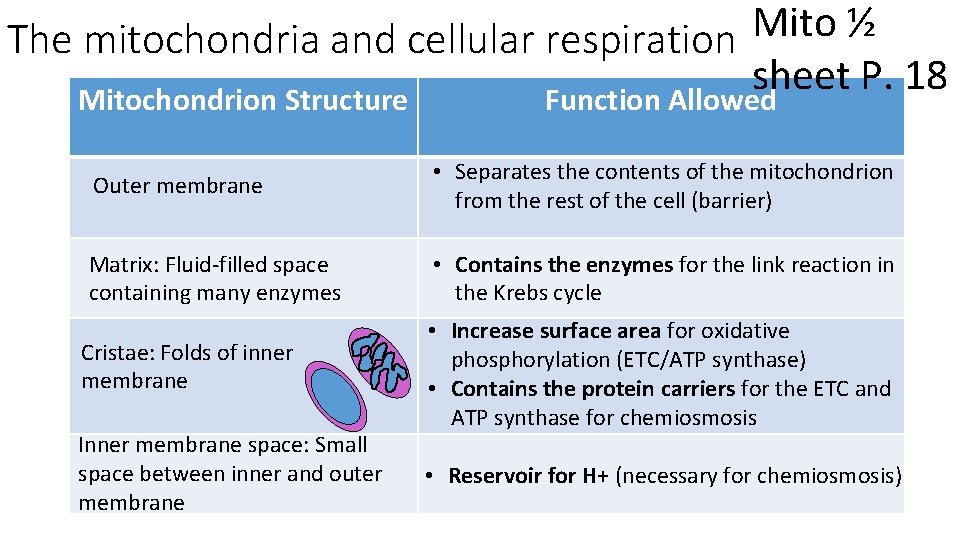
Understandings: • The structure of the mitochondrion is adapted to the function it performs Skill: • Annotation of a diagram of a mitochondrion to indicate the adaptations to its function

Mito ½ The mitochondria and cellular respiration sheet P. 18 Mitochondrion Structure Function Allowed Outer membrane • Separates the contents of the mitochondrion from the rest of the cell (barrier) Matrix: Fluid-filled space containing many enzymes • Contains the enzymes for the link reaction in the Krebs cycle Cristae: Folds of inner membrane Inner membrane space: Small space between inner and outer membrane • Increase surface area for oxidative phosphorylation (ETC/ATP synthase) • Contains the protein carriers for the ETC and ATP synthase for chemiosmosis • Reservoir for H+ (necessary for chemiosmosis)

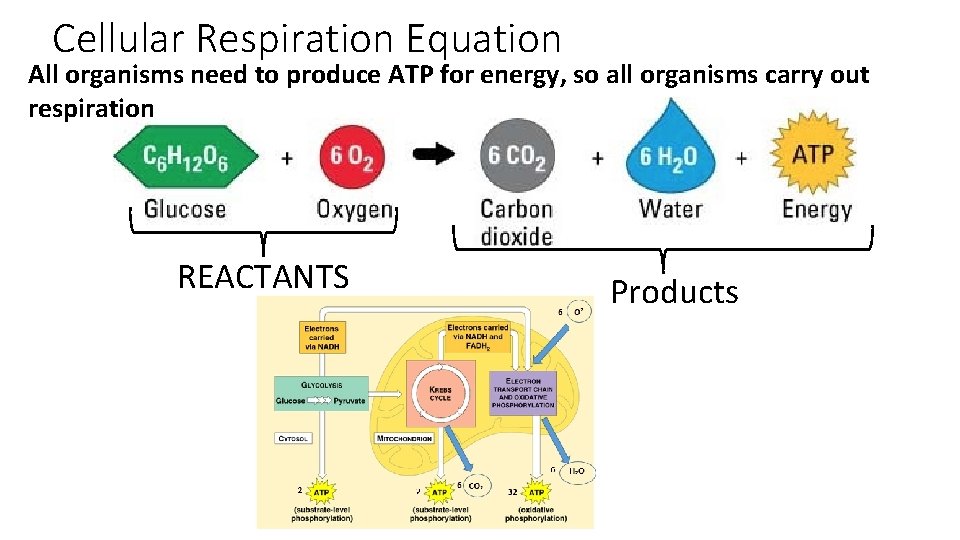
Cellular Respiration Equation All organisms need to produce ATP for energy, so all organisms carry out respiration REACTANTS Products

Crash Course: Cellular Respiration (11 m-13 m 25 s) https: //www. youtube. com/watch? v=00 jb. G_cf. Gu. Q

Cell Respiration Song 4 m 53 s • https: //www. youtube. com/watch? v=3 a. Zrkdzrd 04


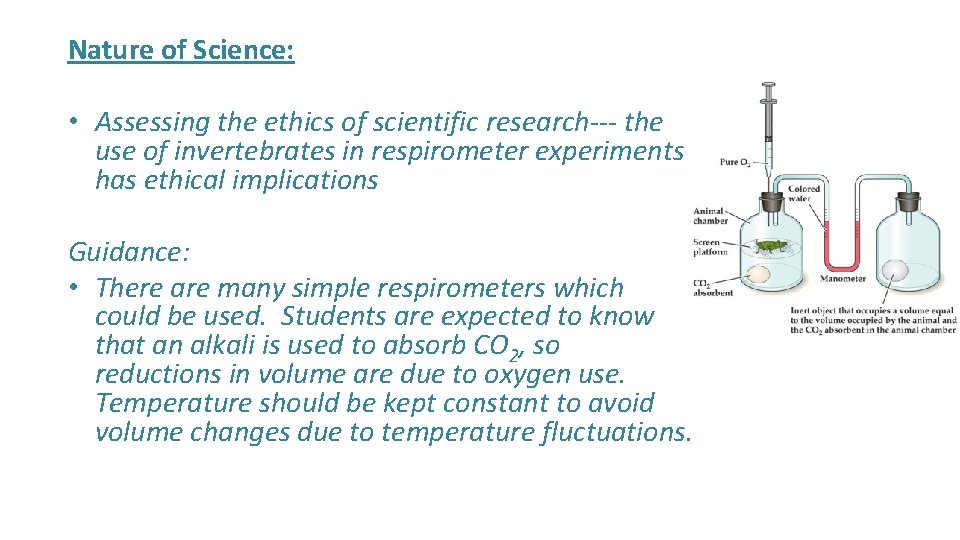
Nature of Science: • Assessing the ethics of scientific research--- the use of invertebrates in respirometer experiments has ethical implications Guidance: • There are many simple respirometers which could be used. Students are expected to know that an alkali is used to absorb CO 2, so reductions in volume are due to oxygen use. Temperature should be kept constant to avoid volume changes due to temperature fluctuations.

Skill: • Analysis of results from experiments involving measurement of respiration rates in germinating seeds or invertebrates using a respirometer.

Respiration Data Analysis • Please complete the worksheet on respirometers and respiration for homework • This will go on notebook p. 22 of your • The following slides are for support, you do NOT need to take notes.

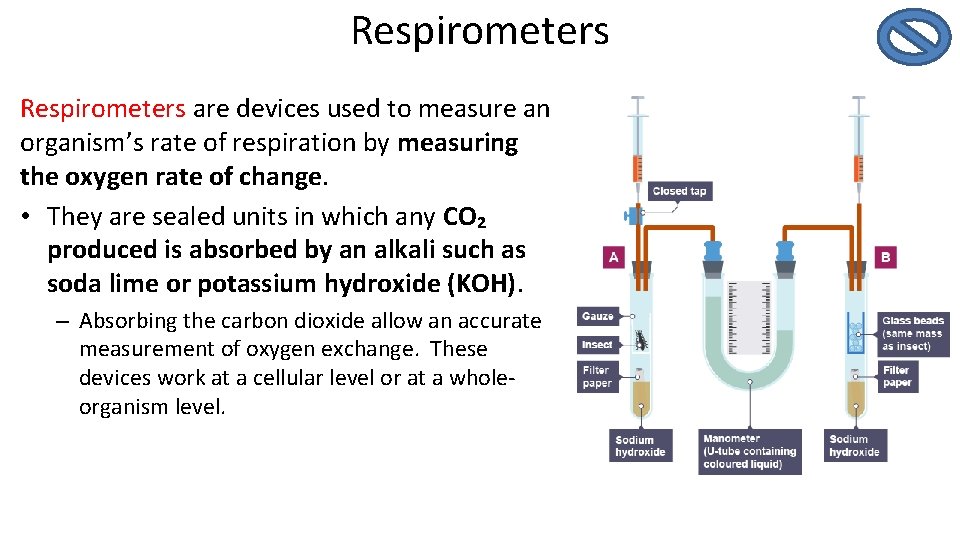
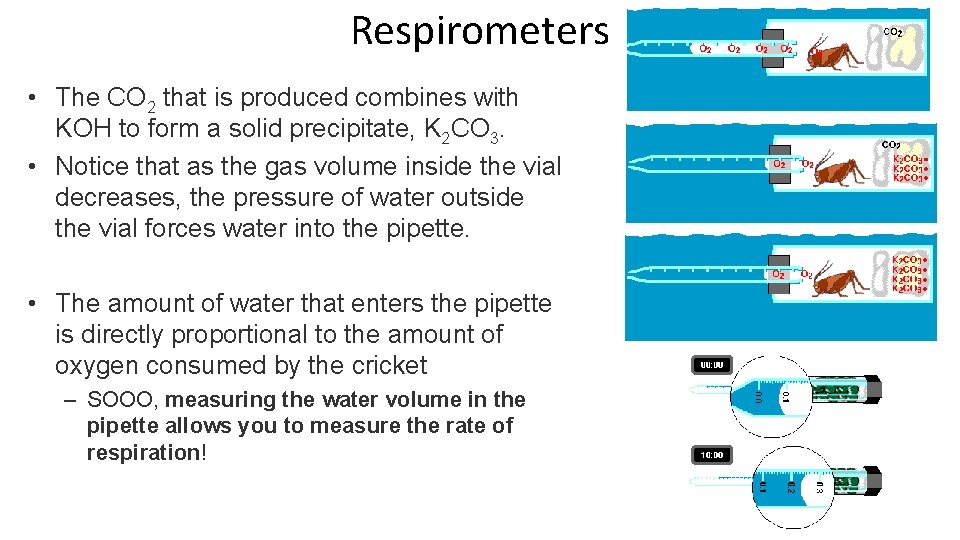
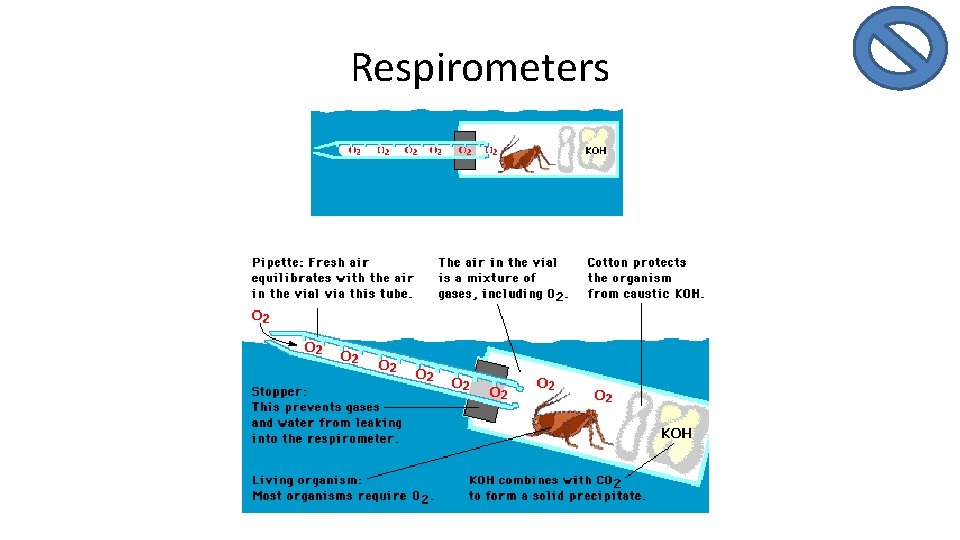
Respirometers are devices used to measure an organism’s rate of respiration by measuring the oxygen rate of change. • They are sealed units in which any CO₂ produced is absorbed by an alkali such as soda lime or potassium hydroxide (KOH). – Absorbing the carbon dioxide allow an accurate measurement of oxygen exchange. These devices work at a cellular level or at a wholeorganism level.

Respirometers • The CO 2 that is produced combines with KOH to form a solid precipitate, K 2 CO 3. • Notice that as the gas volume inside the vial decreases, the pressure of water outside the vial forces water into the pipette. • The amount of water that enters the pipette is directly proportional to the amount of oxygen consumed by the cricket – SOOO, measuring the water volume in the pipette allows you to measure the rate of respiration!

Respirometers

Germination: the process by which a plant grows from a seed • Remember key vocab: Anaerobic v. Aerobic

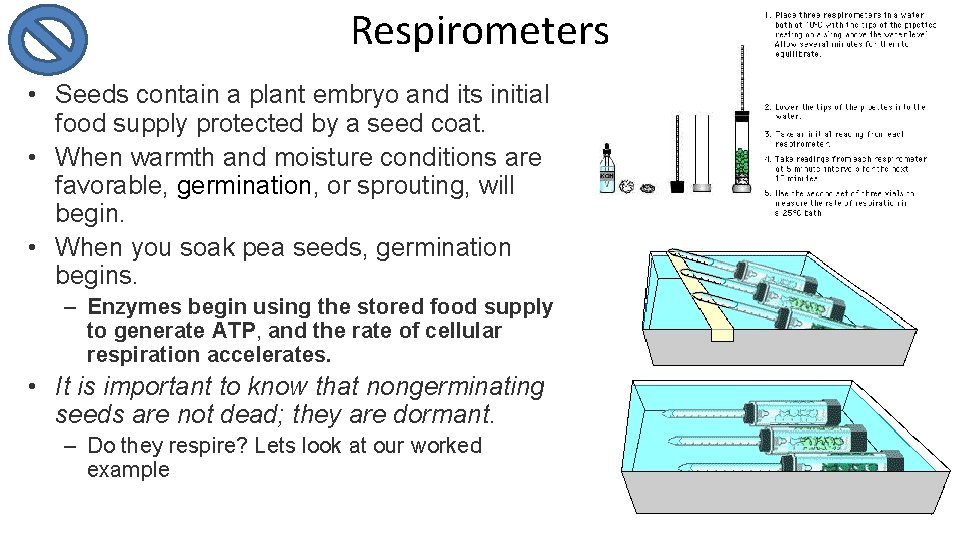
Respirometers • Seeds contain a plant embryo and its initial food supply protected by a seed coat. • When warmth and moisture conditions are favorable, germination, or sprouting, will begin. • When you soak pea seeds, germination begins. – Enzymes begin using the stored food supply to generate ATP, and the rate of cellular respiration accelerates. • It is important to know that nongerminating seeds are not dead; they are dormant. – Do they respire? Lets look at our worked example

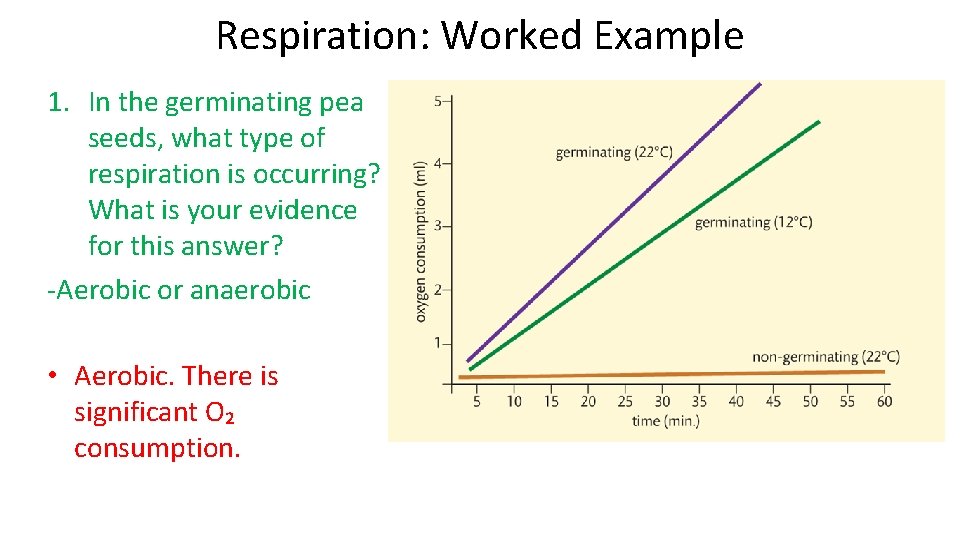
Respiration: Worked Example 1. In the germinating pea seeds, what type of respiration is occurring? What is your evidence for this answer? -Aerobic or anaerobic • Aerobic. There is significant O₂ consumption.

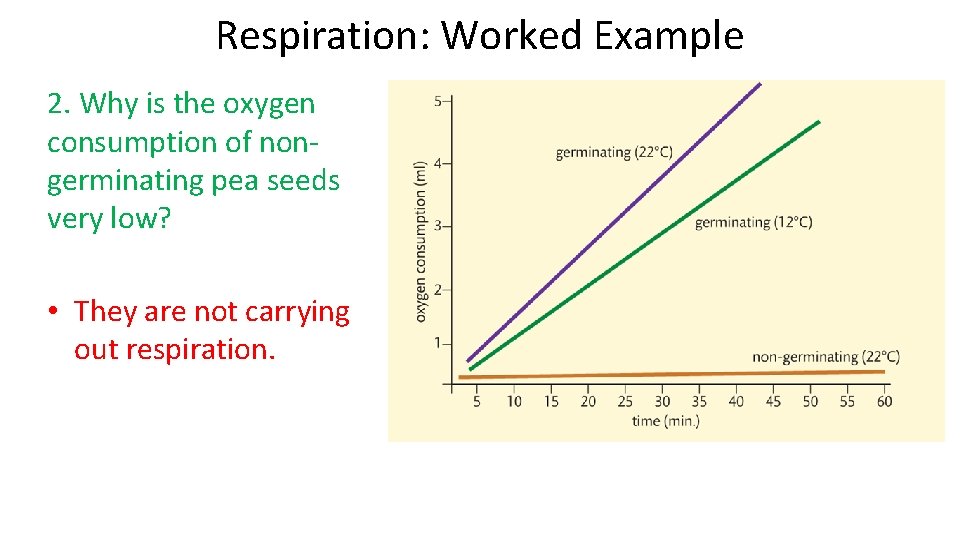
Respiration: Worked Example 2. Why is the oxygen consumption of nongerminating pea seeds very low? • They are not carrying out respiration.

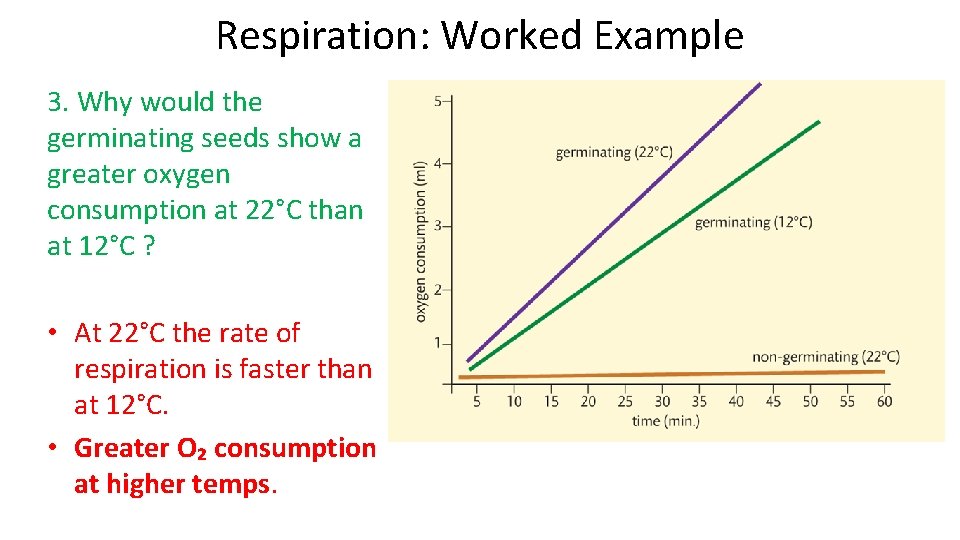
Respiration: Worked Example 3. Why would the germinating seeds show a greater oxygen consumption at 22°C than at 12°C ? • At 22°C the rate of respiration is faster than at 12°C. • Greater O₂ consumption at higher temps.

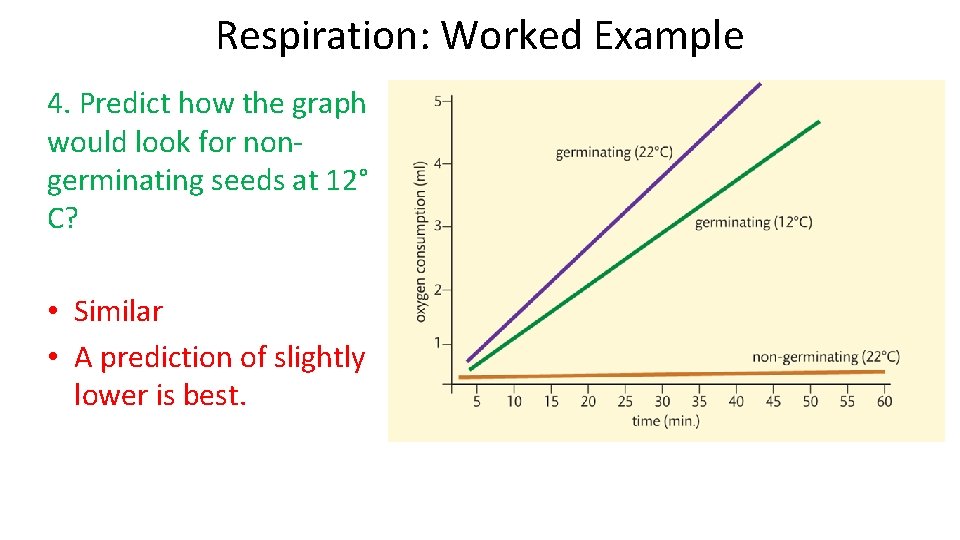
Respiration: Worked Example 4. Predict how the graph would look for nongerminating seeds at 12° C? • Similar • A prediction of slightly lower is best.

Nature of Science: • Peter Mitchell’s chemiosmotic theory encountered years of opposition before it was finally accepted. For what reasons does falsification not always result in an immediate acceptance of new theories or a paradigm shift?

The Chemiosmotic Theory In 1961, Peter Mitchell proposed the chemiosmotic hypothesis to explain the coupling of electron transport in the inner mitochondrial membrane to ATP synthesis • This was a radical idea at the time • Took many years to be accepted • He was awarded the Nobel Prize for Chemistry in 1978