Soil SalinitySodicityAlkalinity and Nutrients Section E SWES 316










































- Slides: 57

Soil Salinity/Sodicity/Alkalinity and Nutrients Section E SWES 316



Definition Salt-Affected Soil: Any soil containing sufficient quantities of soluble salts or sodium to cause adverse effects to plants or soil Saline Sodic Saline-Sodic

Definition Alkaline Soil: A soil with p. H >7. 0. Commonly, soil alkalinity is found in areas with limited soil weathering. Moderately alkaline p. H 7 -8. Often but not always associated with the presence of Ca. CO 3 in soils (calcareous) Highly alkaline p. H >8. Often associated with the presence of excess exchangeable Na in soils.

Soil Salinity Soils in arid regions commonly have “excessive” concentrations of soluble salts because: Lack of leaching to remove salts Poor Drainage Salts added in irrigation water

Soluble Salts Common soluble cations found in saline soils: Ca 2+ Mg 2+ Na+ K+ Common soluble anions found in saline soils: Cl. SO 42 HCO 3 - /CO 32 NO 3 -

Saline Soils Definition: Have an electrical conductivity of a saturated paste extract (ECe) of >4 d. S/m and an exchangeable sodium percentage (ESP) of <15%. So, to classify a soil as saline, the EC, exchangeable Na, and CEC must be known. Note: these numbers are somewhat arbitrary.

Measurements EC is measured in a saturated paste extract. The soil is saturated, extracted and the EC is measured with a conductivity meter.



Properties of Saline Soils Saline soils typically: Are well-aggregated (salts flocculate clays) Have a p. H from 7 to 8 (usually occur in areas of limited soil weathering) Are often calcareous (contain calcium carbonate)

Soil clay particles can be unattached to one another (dispersed) or clumped together (flocculated) in aggregates. Soil aggregates are cemented clusters of sand, silt, and clay particles. Dispersed Particles Flocculated Particles

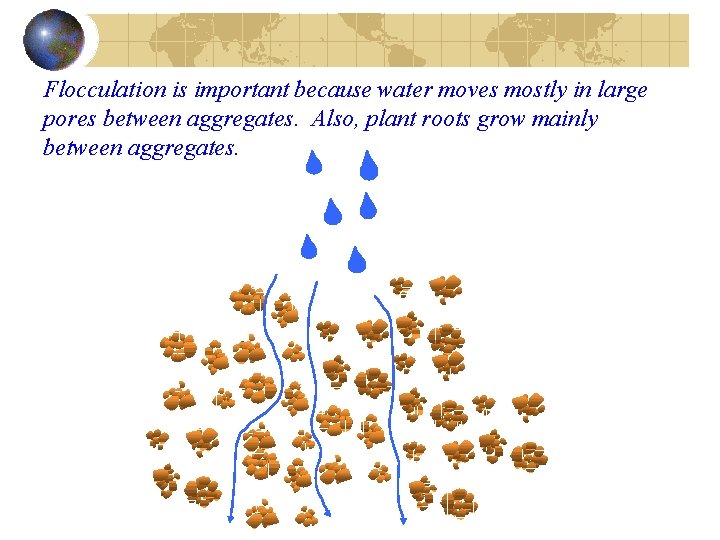
Flocculation is important because water moves mostly in large pores between aggregates. Also, plant roots grow mainly between aggregates.

In all but the sandiest soils, dispersed clays plug soil pores and impede water infiltration and soil drainage.

Most clay particles have a negative electrical charge. Like charges repel, so clay particles repel one another. Negatively charged clay particle

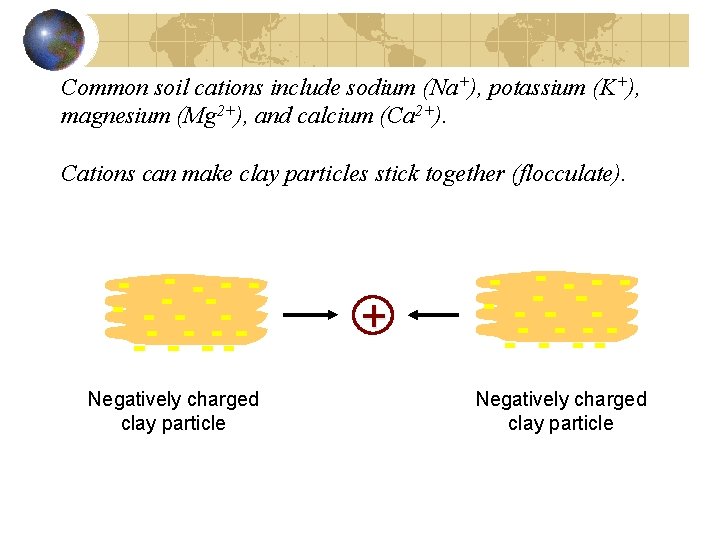
Common soil cations include sodium (Na+), potassium (K+), magnesium (Mg 2+), and calcium (Ca 2+). Cations can make clay particles stick together (flocculate). + Negatively charged clay particle

Flocculating Cations We can divide cations into two categories Poor flocculators • Sodium Good flocculators • Calcium • Magnesium Relative Flocculating Power Ion Sodium Na+ 1. 0 Potassium K+ 1. 7 Magnesium Mg 2+ 27. 0 Calcium Ca 2+ 43. 0 Sumner and Naidu, 1998

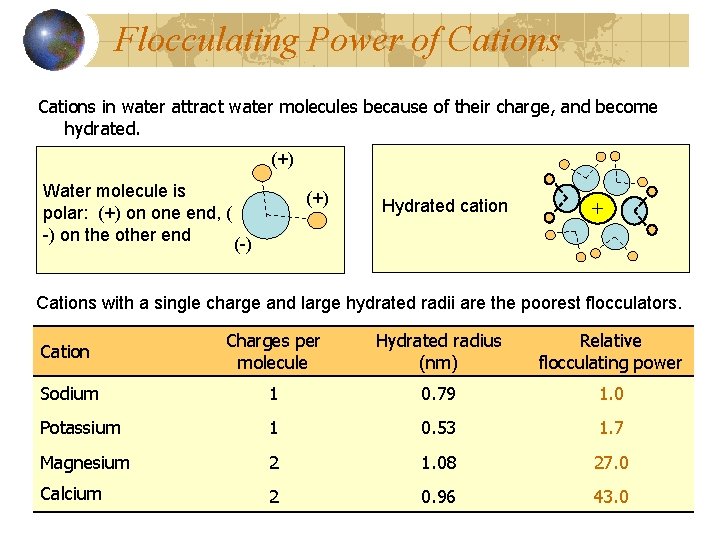
Flocculating Power of Cations in water attract water molecules because of their charge, and become hydrated. (+) Water molecule is polar: (+) on one end, ( -) on the other end (+) Hydrated cation + (-) Cations with a single charge and large hydrated radii are the poorest flocculators. Cation Charges per molecule Hydrated radius (nm) Relative flocculating power Sodium 1 0. 79 1. 0 Potassium 1 0. 53 1. 7 Magnesium 2 1. 08 27. 0 Calcium 2 0. 96 43. 0

Dispersion Clay with only exchangeable Ca 2+, clay particles can approach closely, promoting flocculation Ca 2+ Na+ Clay with exchangeable Ca 2+ and Na+, clay particles cannot approach closely, causing dispersion

Sodium Adsorption Ratio The ratio of ‘bad’ to ‘good’ flocculators gives an indication of the relative status of these cations: + + + ++ ++ Na+ Ca 2+ and Mg 2+ Mathematically, this is expressed as the ‘sodium adsorption ratio’ or SAR: [Na+] SAR = [Ca 2+] + [Mg 2+] where concentrations are expressed in mmoles/L

Exchangeable Sodium Percentage AN alternative to SAR is ESP, Exchangeable Sodium Percentage + ++ + - - - - ++ ++ ++ Na+ Ca 2+ and Mg 2+ Mathematically, this is expressed as the percentage of the CEC (cation exchange capacity) that is filled with sodium in units of charge per mass (cmol(+)/kg) Na+ ESP = Cation Exchange Capacity SAR and ESP are approximately equal numerically

Electrical Conductivity Ions in solution conduct electricity, so the total amount of soluble soil ions can be estimated by measuring the electrical conductivity (EC) of a soil water extract. EC is measured in units of conductance over a known distance: deci-Siemens per meter or d. S/m Soil with a high EC is salty; soil with a low EC is not.

Aggregate stability (dispersion and flocculation) depends on the balance (SAR) between (Ca 2+ and Mg 2+) and Na+ as well as the amount of soluble salts (EC) in the soil. Na+ Ca 2+ and Mg 2+ ++ ++ ++ + + + + SAR EC Lower EC Flocculated soil Higher EC Dispersed soil

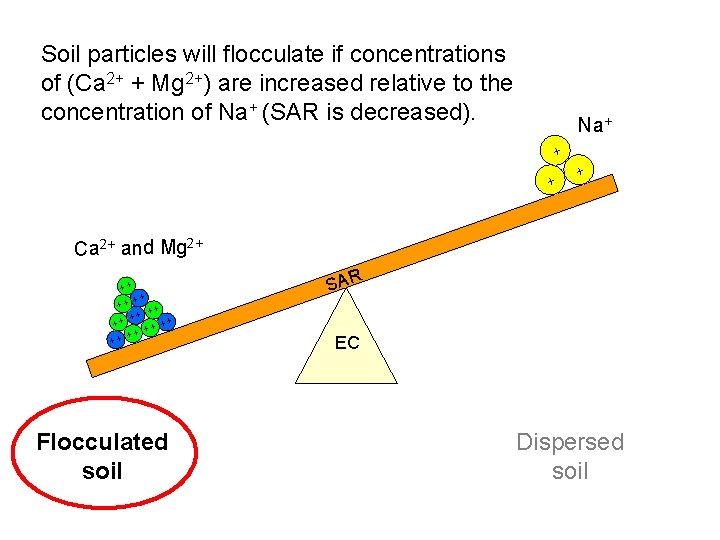
Soil particles will flocculate if concentrations of (Ca 2+ + Mg 2+) are increased relative to the concentration of Na+ (SAR is decreased). Na+ + Ca 2+ and Mg 2+ ++ ++ + + Flocculated soil SAR EC Dispersed soil

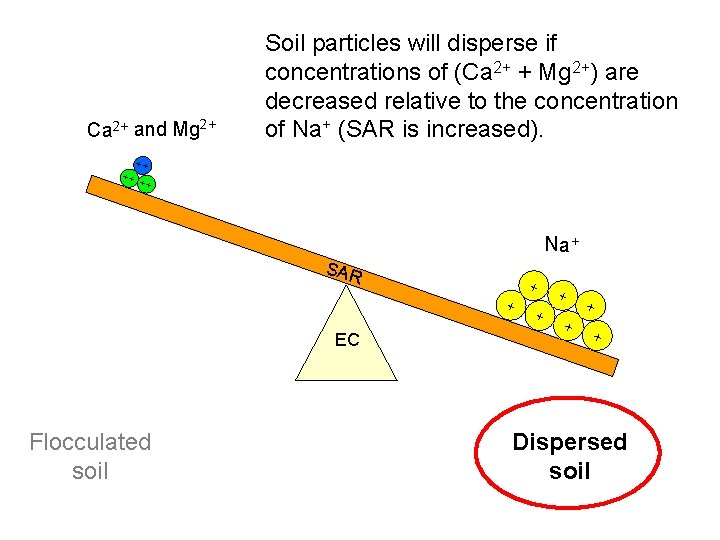
Ca 2+ and Mg 2+ Soil particles will disperse if concentrations of (Ca 2+ + Mg 2+) are decreased relative to the concentration of Na+ (SAR is increased). ++ ++ + + Na+ SAR + + EC Flocculated soil + + + Dispersed soil

Soil particles will flocculate if the amount of soluble salts in the soil is increased (increased EC), even if there is a lot of sodium. Na+ + + + Ca 2+ and Mg 2+ ++ ++ + + Flocculated soil SAR EC Lower EC Higher EC Dispersed soil

Ca 2+ and Mg 2+ Soil particles may disperse if the amount of soluble salts in the soil is decreased (i. e. if EC is decreased). ++ ++ ++ Na+ SAR + EC Lower EC Flocculated soil + + Higher EC Dispersed soil

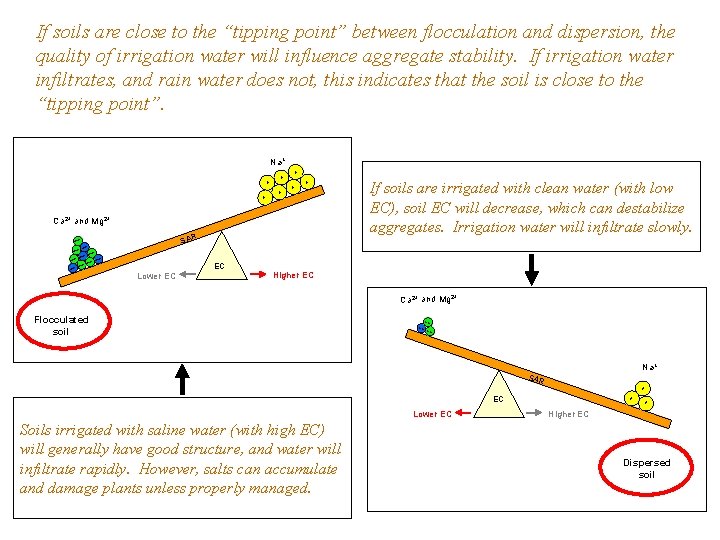
If soils are close to the “tipping point” between flocculation and dispersion, the quality of irrigation water will influence aggregate stability. If irrigation water infiltrates, and rain water does not, this indicates that the soil is close to the “tipping point”. Na+ + + + Ca 2+ and Mg 2+ SAR ++ ++++ If soils are irrigated with clean water (with low EC), soil EC will decrease, which can destabilize aggregates. Irrigation water will infiltrate slowly. ++ ++ + ++ Lower EC EC Higher EC Ca 2+ and Mg 2+ Flocculated soil ++ ++ ++ Na+ SAR + EC Lower EC Soils irrigated with saline water (with high EC) will generally have good structure, and water will infiltrate rapidly. However, salts can accumulate and damage plants unless properly managed. + + Higher EC Dispersed soil

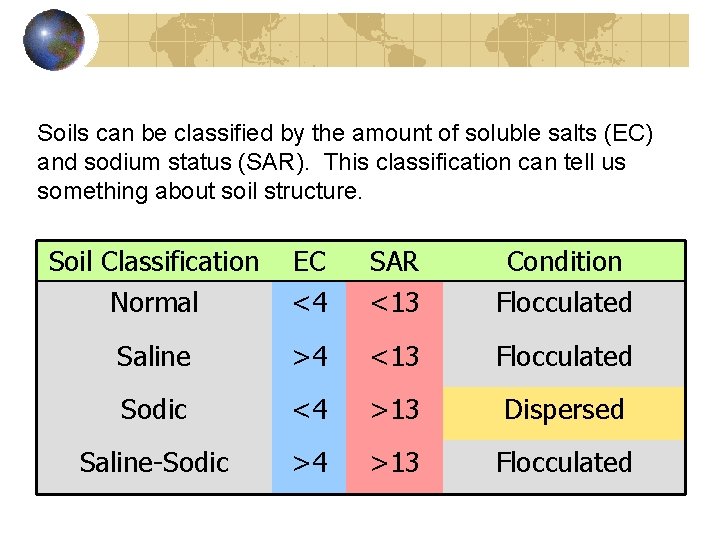
Soils can be classified by the amount of soluble salts (EC) and sodium status (SAR). This classification can tell us something about soil structure. Soil Classification Normal EC <4 SAR <13 Condition Flocculated Saline >4 <13 Flocculated Sodic <4 >13 Dispersed Saline-Sodic >4 >13 Flocculated

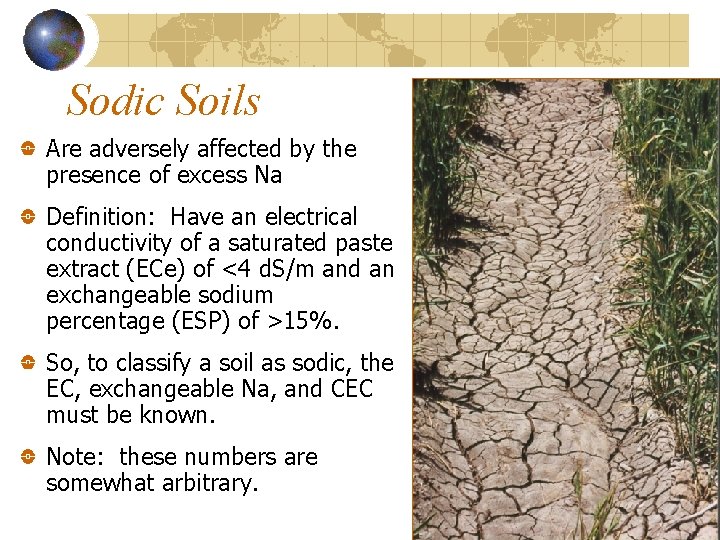
Sodic Soils Are adversely affected by the presence of excess Na Definition: Have an electrical conductivity of a saturated paste extract (ECe) of <4 d. S/m and an exchangeable sodium percentage (ESP) of >15%. So, to classify a soil as sodic, the EC, exchangeable Na, and CEC must be known. Note: these numbers are somewhat arbitrary.

Properties of Sodic Soils Sodic soils typically: Are poorly-aggregated (sodium disperses clays) Have slow rates of water infiltration Have a p. H of 8 or above. This is due to the presence of soluble Na 2 CO 3.

Saline-Sodic Soils Definition: Have an electrical conductivity of a saturated paste extract (ECe) of >4 d. S/m and an exchangeable sodium percentage (ESP) of >15%. So, to classify a soil as saline-sodic, the EC, exchangeable Na, and CEC must be known.

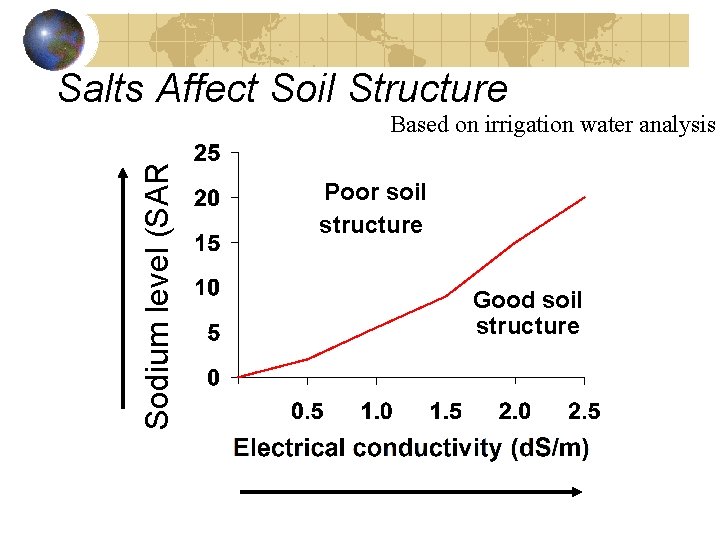
Salts Affect Soil Structure Sodium level (SAR) Based on irrigation water analysis Poor soil structure Good soil structure

Salt and Sodium Risks Salinity is mostly harmful to plant growth. Most plants, especially crop plants, are sensitive to salts. The properties of the soils themselves can be improved by the presence of salts (flocculation). Sodium is harmful to plants and soils Sodium causes soils to have undesirable physical and chemical properties. Sodium can also cause toxicities to plants. Alkaline p. H (esp. in sodic soils) can limit nutrient availability to plants.

Salt Effects on Plants Excess soluble salts can be harmful to plant growth because: Salts lower the osmotic potential energy of soil water. Water is less available to plants. Some soluble salt ions can have specific toxic effects on plants, such as: • Na+, Cl- , H 3 BO 3



Soil Salinity and Nutrients Some specific effects of salinity on nutrients: High Na concentrations can inhibit Ca, Mg uptake by roots. Ion toxicity limits nutrient uptake, lowering nutrient requirements. High HCO 3 - can limit Ca availability.


Soil Alkalinity and Nutrients Soil p. H >7. 5 Alkalinity is specifically associated with: Sodic soils Calcareous soils Soils high in soluble carbonates Saline soils may or may not be alkaline.

Soil Alkalinity and Nutrients Specific Effects: p. H dependent AEC decreases, and CEC increases as p. H increases. Nintrogen: NH 3 volatilization increases as p. H increases. Phosphorus: P availability decreases at p. H>6 due to Ca-P reactions. Fe, Mn, Cu, Zn: solubility decreases 10 -100 x for every 1 p. H unit increase. B: availability decreases at p. H >7.

Treatment for Saline Soils Amendments for removing salts from soils: Nothing Management Practices Adequate Leaching Maintain soil drainage through proper tillage


Soil Amendments for Salinity and Sodium Control § Soil amendments will not help with salinity control unless a sodium problem also exists. § Amendment additions are necessary to correct sodium problems. Leaching alone is not enough.

Should Alkaline Soils be Acidified? It is rarely advisable to acidify soils to significantly lower p. H: Amounts required may be enormous: A soil with 2% Ca. CO 3 in the top 30 cm will contain 84000 kg Ca. CO 3/ha. This would require about 93 tons H 2 SO 4/ha to neutralize the Ca. CO 3. There is rarely an economic benefit to such large application rates.

So, what to do about alkaline soils? If soils are sodic and highly alkaline, use of gypsum and leaching will usually lower p. H to <8. 4. When p. H is <8. 4, micronutrient deficiencies in most crops are rare and manageable with foliar applications.


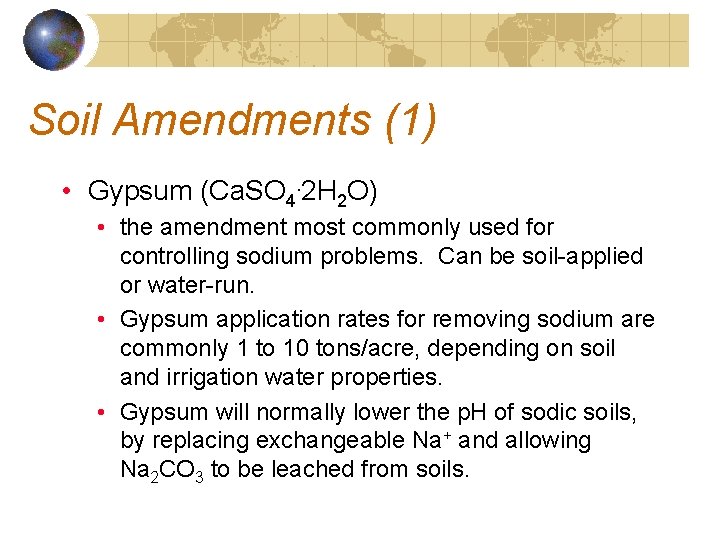
Soil Amendments (1) • Gypsum (Ca. SO 4. 2 H 2 O) • the amendment most commonly used for controlling sodium problems. Can be soil-applied or water-run. • Gypsum application rates for removing sodium are commonly 1 to 10 tons/acre, depending on soil and irrigation water properties. • Gypsum will normally lower the p. H of sodic soils, by replacing exchangeable Na+ and allowing Na 2 CO 3 to be leached from soils.

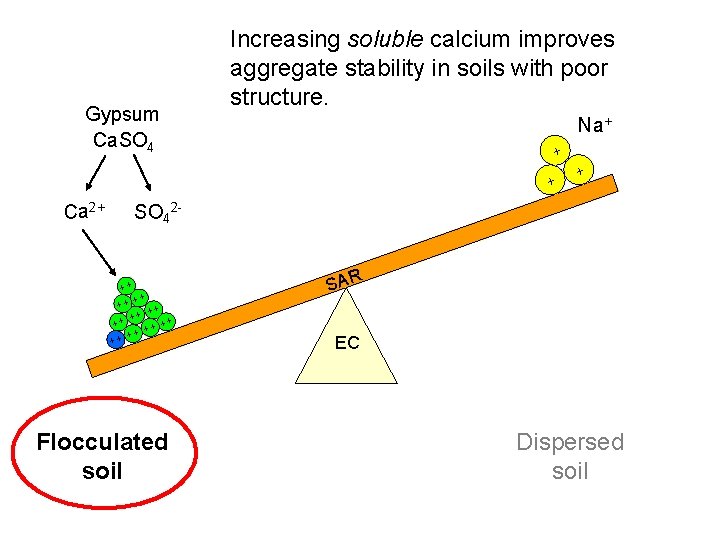
Gypsum Ca. SO 4 Increasing soluble calcium improves aggregate stability in soils with poor structure. Na+ + + Ca 2+ + SO 42 - ++ + + ++ ++ + + Flocculated soil SAR EC Dispersed soil

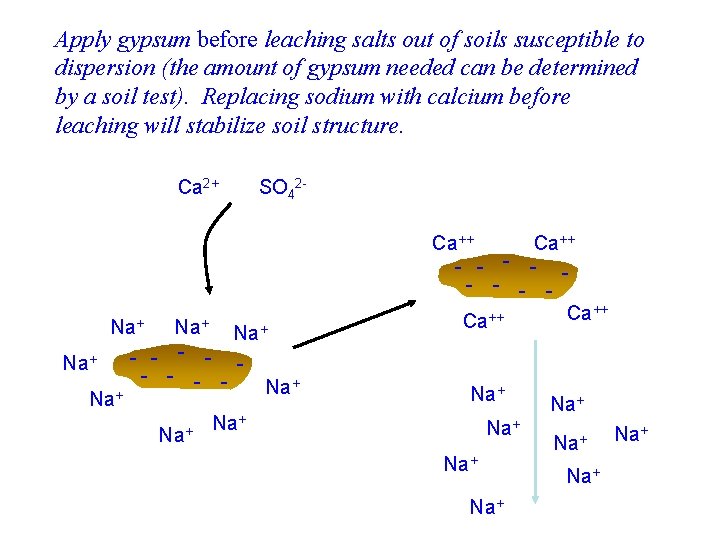
Apply gypsum before leaching salts out of soils susceptible to dispersion (the amount of gypsum needed can be determined by a soil test). Replacing sodium with calcium before leaching will stabilize soil structure. Ca 2+ SO 42 - Na+ Na+ - - - Na+ + Na Ca++ - - - - Ca++ Na+ Na+

Gypsum Application

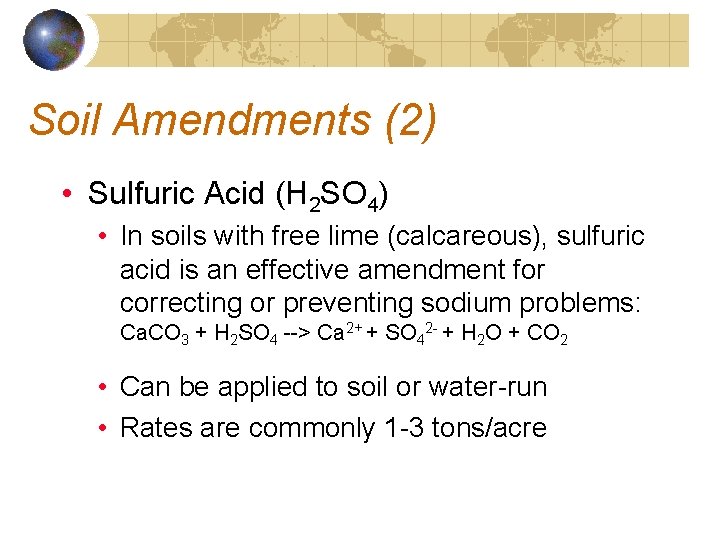
Soil Amendments (2) • Sulfuric Acid (H 2 SO 4) • In soils with free lime (calcareous), sulfuric acid is an effective amendment for correcting or preventing sodium problems: Ca. CO 3 + H 2 SO 4 --> Ca 2+ + SO 42 - + H 2 O + CO 2 • Can be applied to soil or water-run • Rates are commonly 1 -3 tons/acre

Sulfuric acid* can be used instead of gypsum on calcareous (Ca. CO 3 containing) soil only. Sulfuric acid dissolves calcium carbonate in the soil and makes gypsum! *Sulfuric acid is extremely dangerous and should only be handled trained personnel. by

Constant H 2 SO 4 injection keeps water p. H low and prevents formation of Ca. CO 3 in the drip lines, and also dissolves some Ca. CO 3 in the soil, helping to maintain high exchangeable Ca 2+ and low exchangeable Na+.

Soil Amendments (3) • Elemental Sulfur 97% Sulfur • Effective acid-forming amendment: soil microorganisms use S to produce sulfuric acid. The sulfuric acid reacts with Ca. CO 3 to release Ca. • Requires microbial activity to react. May take months to react completely. • Reaction: • 2 S + 3 O 2 + 2 H 2 O 2 H 2 SO 4

Elemental sulfur can also be used as an alternative to gypsum on calcareous soils Soil microbes convert sulfur into sulfuric acid H 2 SO 4 dissolves calcium carbonate and makes gypsum • Conversion to sulfuric acid takes time – several weeks – faster in warm soils