Separation Techniques Methods of Separating Mixtures Magnet Filter







- Slides: 22

Separation Techniques

Methods of Separating Mixtures • • • Magnet Filter Decant Evaporation Distillation

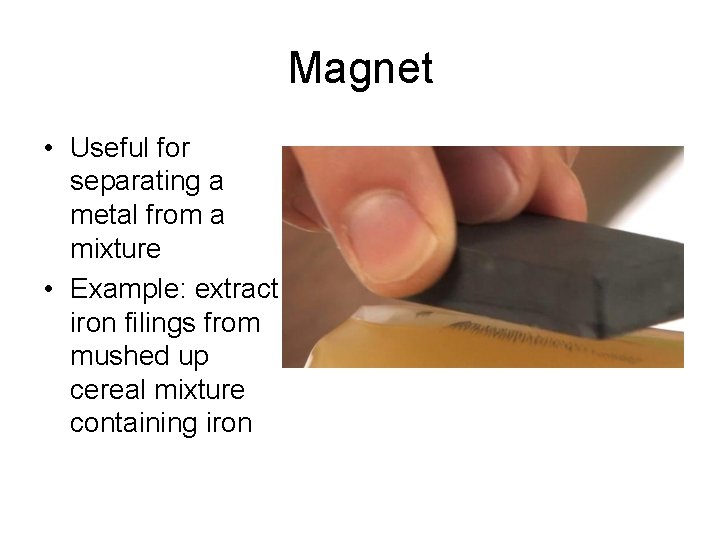
Magnet • Useful for separating a metal from a mixture • Example: extract iron filings from mushed up cereal mixture containing iron

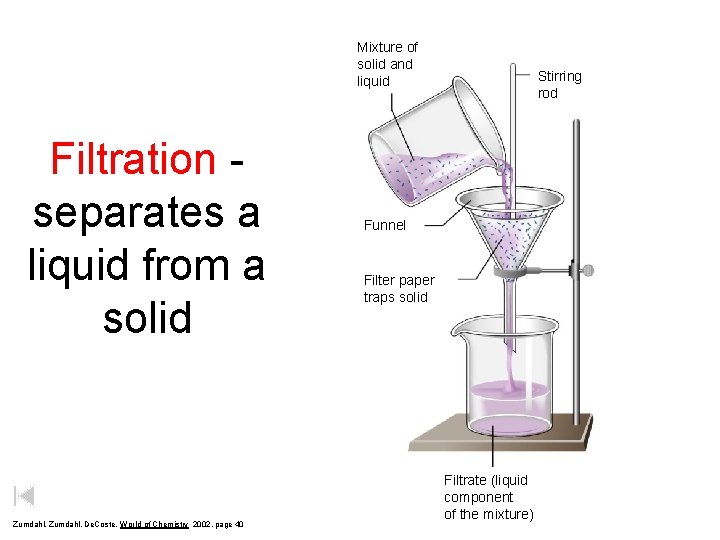
Mixture of solid and liquid Filtration separates a liquid from a solid Zumdahl, De. Coste, World of Chemistry 2002, page 40 Stirring rod Funnel Filter paper traps solid Filtrate (liquid component of the mixture)


Filtration • Separates a liquid from a solid • Use proper size of filter paper for the funnel • Fold filter paper properly • Use distilled water to help keep filter paper open in funnel • Make sure mixture is well stirred • Use glass rod to guide liquid while pouring • Useful for quantitative analysis

Decanting • Also useful for separating a solid from a liquid • Simply pouring the top layer of liquid into another container and leaving the solid behind • To avoid losing any solid some liquid may need to be left behind • Useful when quantitative study is NOT required

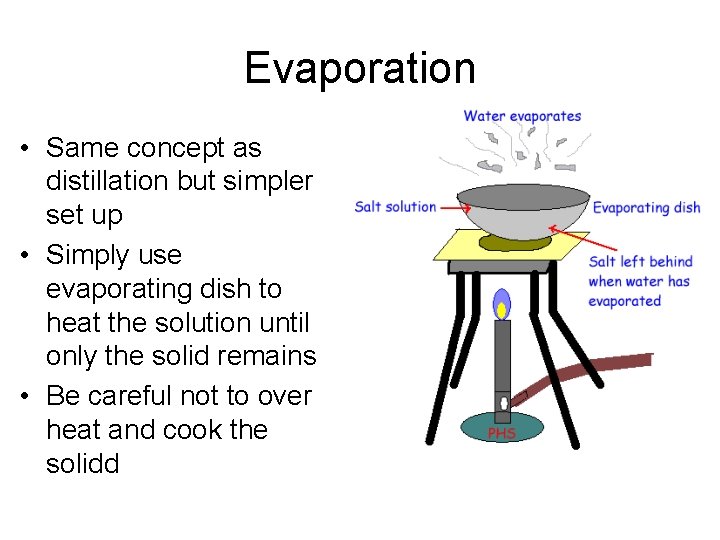
Evaporation • Same concept as distillation but simpler set up • Simply use evaporating dish to heat the solution until only the solid remains • Be careful not to over heat and cook the solidd

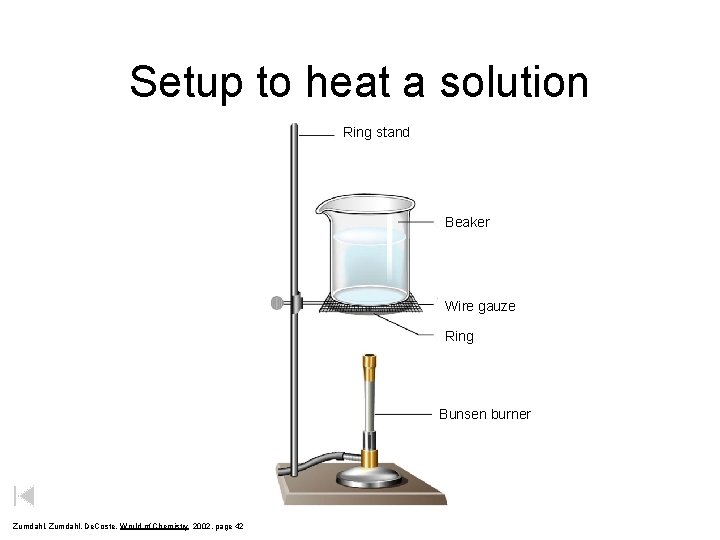
Setup to heat a solution Ring stand Beaker Wire gauze Ring Bunsen burner Zumdahl, De. Coste, World of Chemistry 2002, page 42

Distillation Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.

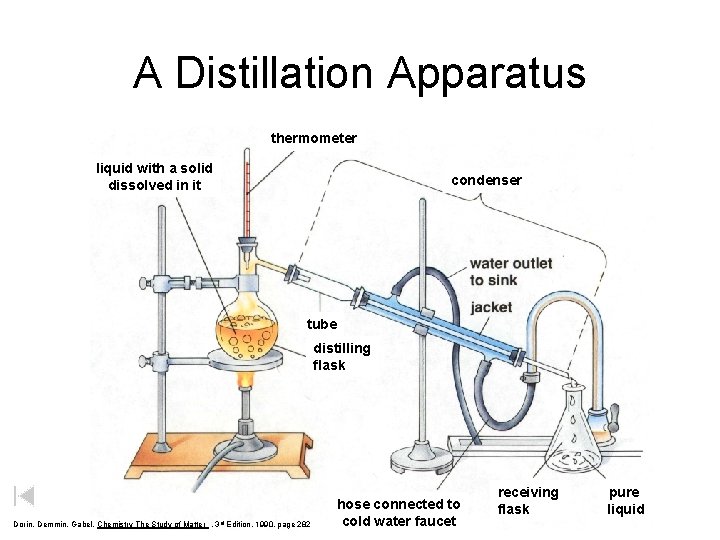
A Distillation Apparatus thermometer liquid with a solid dissolved in it condenser tube distilling flask Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 282 hose connected to cold water faucet receiving flask pure liquid

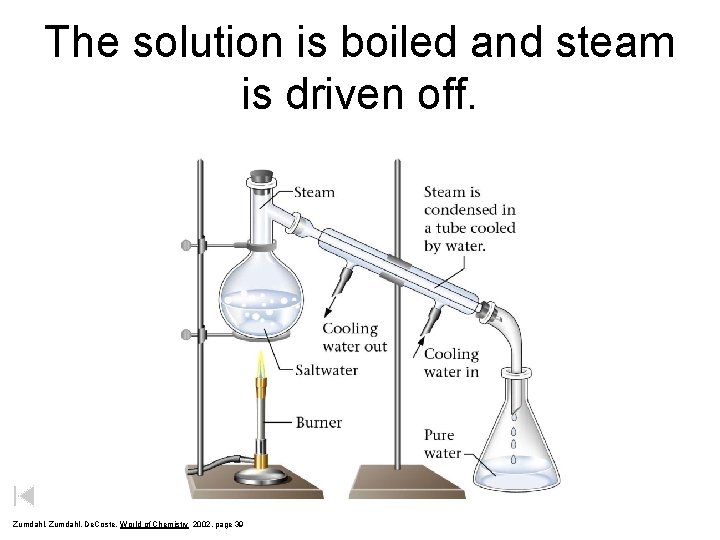
The solution is boiled and steam is driven off. Zumdahl, De. Coste, World of Chemistry 2002, page 39

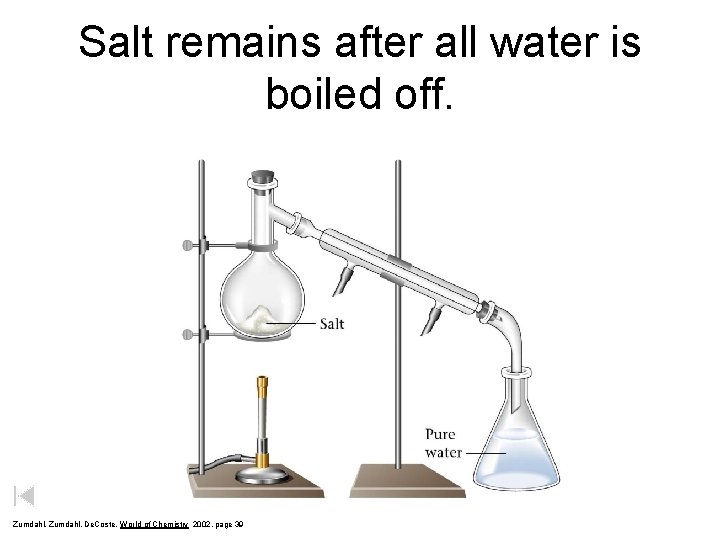
Salt remains after all water is boiled off. Zumdahl, De. Coste, World of Chemistry 2002, page 39

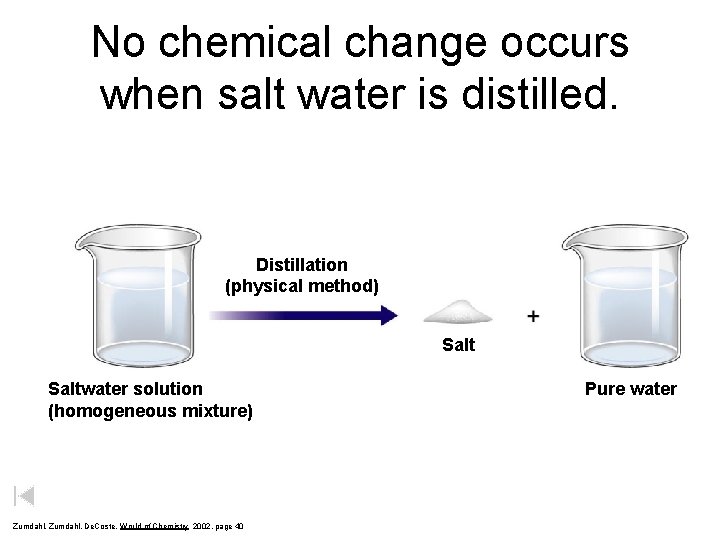
No chemical change occurs when salt water is distilled. Distillation (physical method) Saltwater solution (homogeneous mixture) Zumdahl, De. Coste, World of Chemistry 2002, page 40 Pure water

Electrolysis

Water Molecules Zumdahl, De. Coste, World of Chemistry 2002, page 8

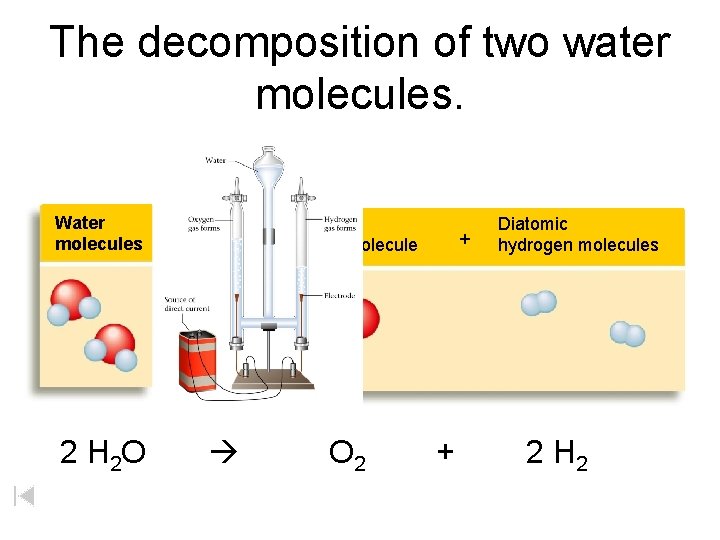
The decomposition of two water molecules. Water molecules Diatomic oxygen molecule + Diatomic hydrogen molecules Electric current 2 H 2 O O 2 + 2 H 2

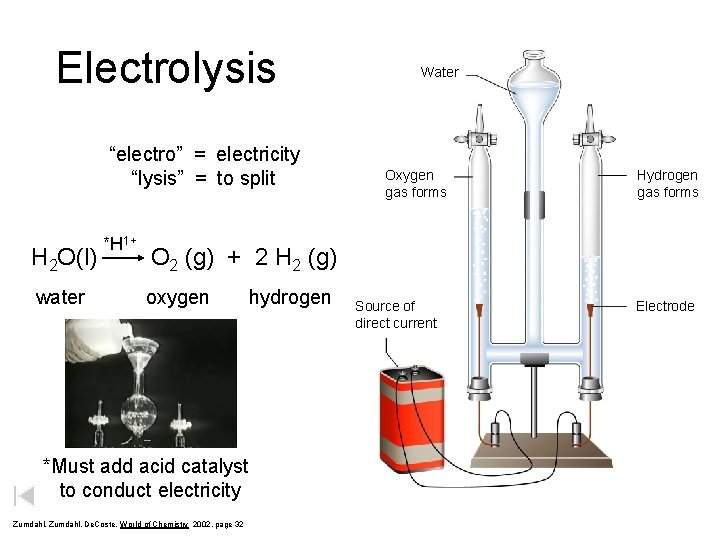
Electrolysis “electro” = electricity “lysis” = to split H 2 O(l) water *H 1+ Water Oxygen gas forms Hydrogen gas forms O 2 (g) + 2 H 2 (g) oxygen *Must add acid catalyst to conduct electricity Zumdahl, De. Coste, World of Chemistry 2002, page 32 hydrogen Source of direct current Electrode

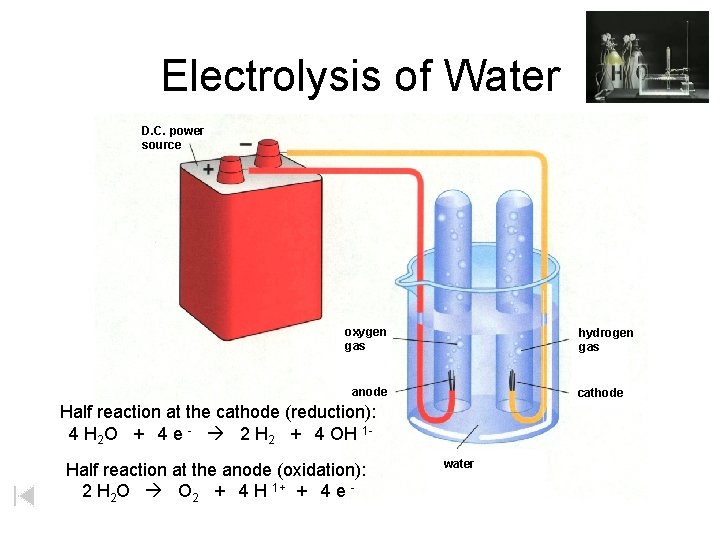
Electrolysis of Water D. C. power source oxygen gas hydrogen gas anode cathode Half reaction at the cathode (reduction): 4 H 2 O + 4 e - 2 H 2 + 4 OH 1 Half reaction at the anode (oxidation): 2 H 2 O O 2 + 4 H 1+ + 4 e - water

Steps to Separate a mixture • Begin with a mixture of sand salt • What are the steps you would follow to separate the sand the salt • You want to end up with pure dry salt and dry sand with no salt left in it

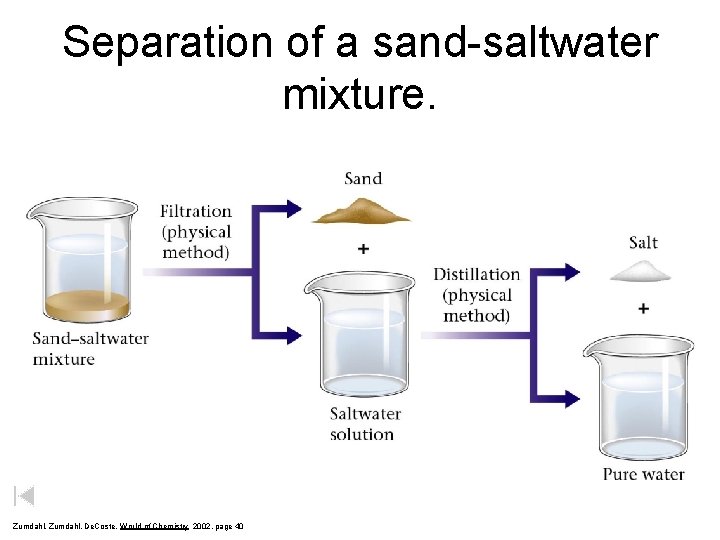
Separation of a sand-saltwater mixture. Zumdahl, De. Coste, World of Chemistry 2002, page 40

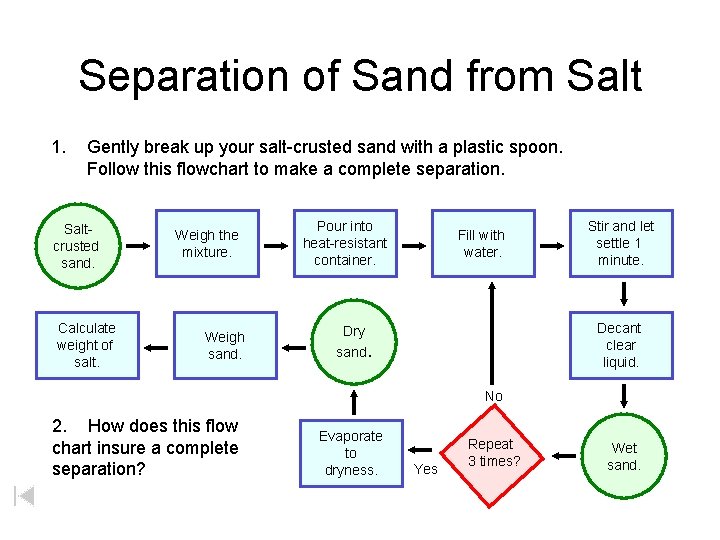
Separation of Sand from Salt 1. Gently break up your salt-crusted sand with a plastic spoon. Follow this flowchart to make a complete separation. Saltcrusted sand. Calculate weight of salt. Weigh the mixture. Weigh sand. Pour into heat-resistant container. Fill with water. Stir and let settle 1 minute. Decant clear liquid. Dry sand. No 2. How does this flow chart insure a complete separation? Evaporate to dryness. Yes Repeat 3 times? Wet sand.