Saturated vs Unsaturated Saturated Contains the maximum amount
















- Slides: 21

Saturated vs Unsaturated

Saturated - Contains the maximum amount of dissolved solute for a given amount of solvent at a specific temperature and pressure.

Unsaturated - Contains less dissolved solute for a given temperature and pressure than a saturated solution.

Supersaturated - Contains more dissolved solute than a saturated solution at the same temperature.

Supersaturated Sodium Acetate Supersaturated Solution

Water as a mixture • Muddy water not a solution. • Muddy water is heterogeneous because it contains larger particles of soil or plant debris.

Water and solutions • Solutions exist in every phase; solid, liquid, or gas. • Solutions of two or more solids are called alloys. • Steel is an alloy (solution) of iron and carbon.

Suspensions • In a mixture called a suspension the particles can range widely in size. • Muddy water, a suspension, will settle when it is left still for a period of time.

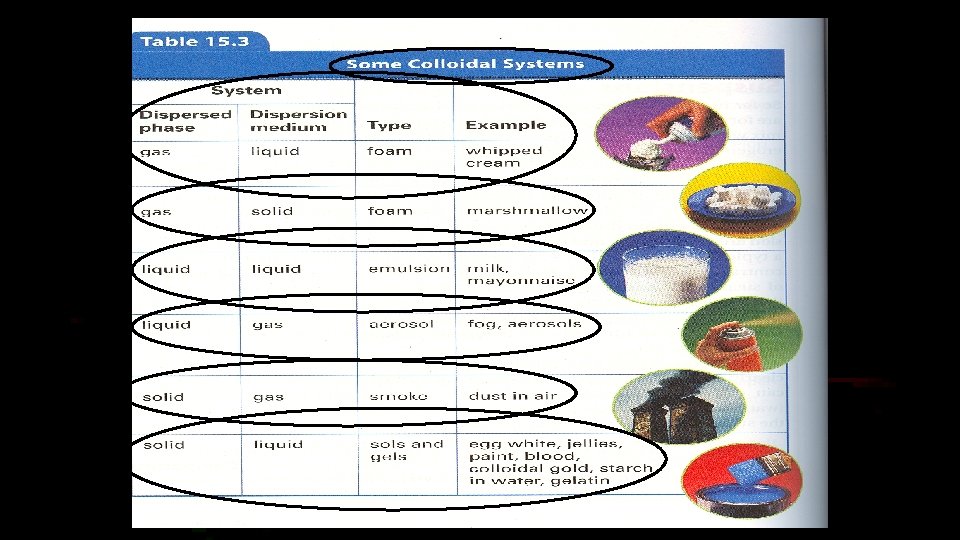
Colloids • Colloids are mixtures, and look like solutions, but their particles are too small to settle to the bottom of their container over time. • Examples of colloids are mayonnaise, egg whites, and gelatin.


Tyndall effect • Tyndall effect is occurring if you shine a flashlight through a jar of liquid and see the light beam.

Separating Mixtures 12

How do we separate …? 13

• Filtration • Centrifuging • Decantation • Evaporating • Crystallization • Distilling • Chromatography • Sieving 14

Filtration - Used when separating a solid substance from a fluid (a liquid or a gas) by passing a mixture through a porous material such as a type of filter. 15

16

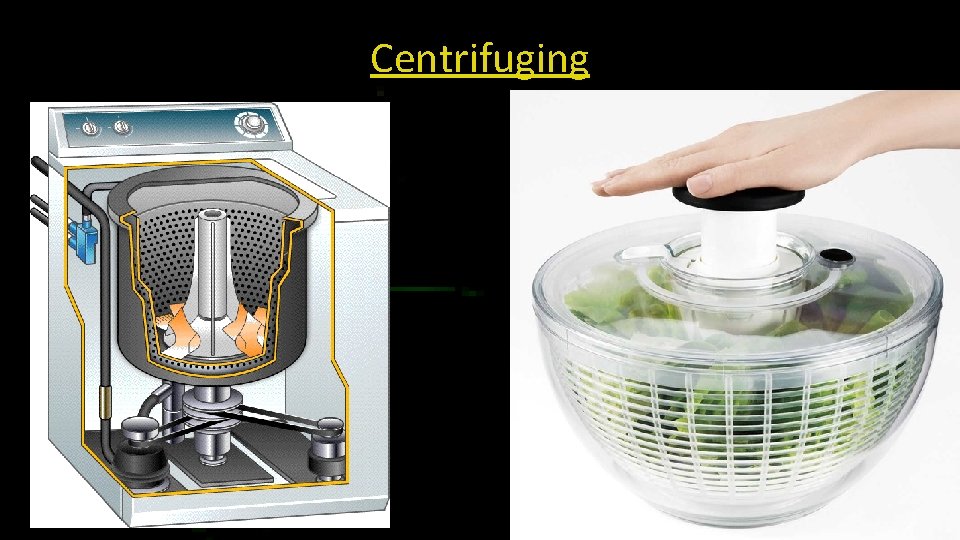
Centrifuges • Centrifuges rotate containers of liquids to separate suspended materials with different densities. • Example: The spin drier in washing machines.

Centrifuging 18

Decanting Decantation is the process of separating a liquid from a solid.

Decantation Wine

Paper Chromatography Separates dissolved chemical substances Place a sample spot on the filter paper Immerse the paper in a water (solvent) The solvent penetrates the paper by capillary action and, in passing over the sample spot, carries along with it the various components of the sample. Separation of the components is brought about by differences in their relative solubility's in the two solvents.