Monday 4 18 and Tues 4 19 Types









- Slides: 17

Monday 4 -18 and Tues 4 -19 Types of Solutions and Solubility Curves Mrs. Wilson

Objectives • Distinguish between solutions, colloids, and suspensions; electrolytes and non-electrolytes; and strong / weak electrolytes. • Distinguish between solutions based on their degree of saturation: saturated, supersaturated, and unsaturated solutions. • Interpret solubility curves for different ionic compounds to determine the degree of saturation of a solution. Homework: Colors of Chemistry Lab due next class. We’ll finish the lab questions next class.

Daily Quiz • You do not need a calculator. You do need a periodic table.

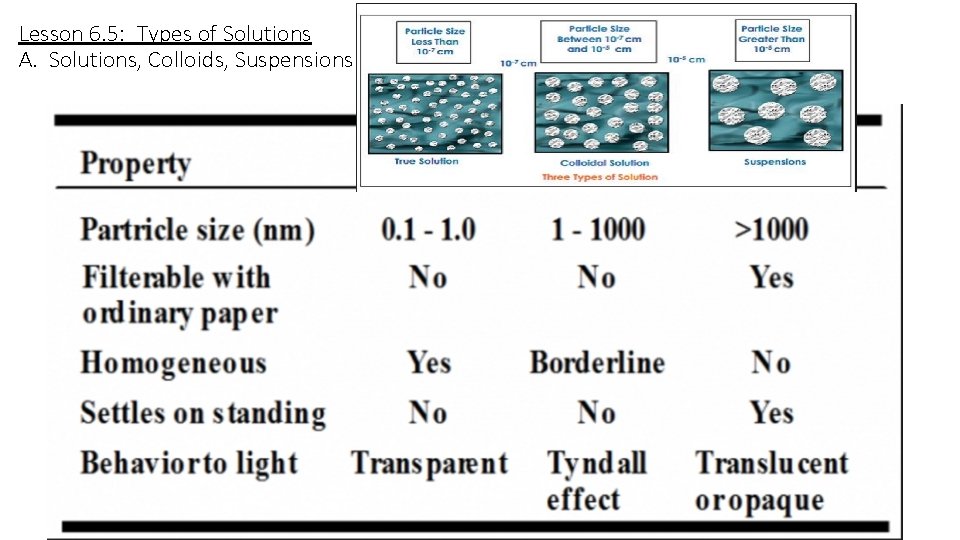
Lesson 6. 5: Types of Solutions A. Solutions, Colloids, Suspensions

Lesson 6. 5: Types of Solutions A. Solutions, Colloids, Suspensions Solution Colloid Tyndall effect: shown only in colloids – the colloidal particles are larger than the wavelength of the incident light, so that light beam “scatters” or broadens in width. Think of car headlights shining through fog.

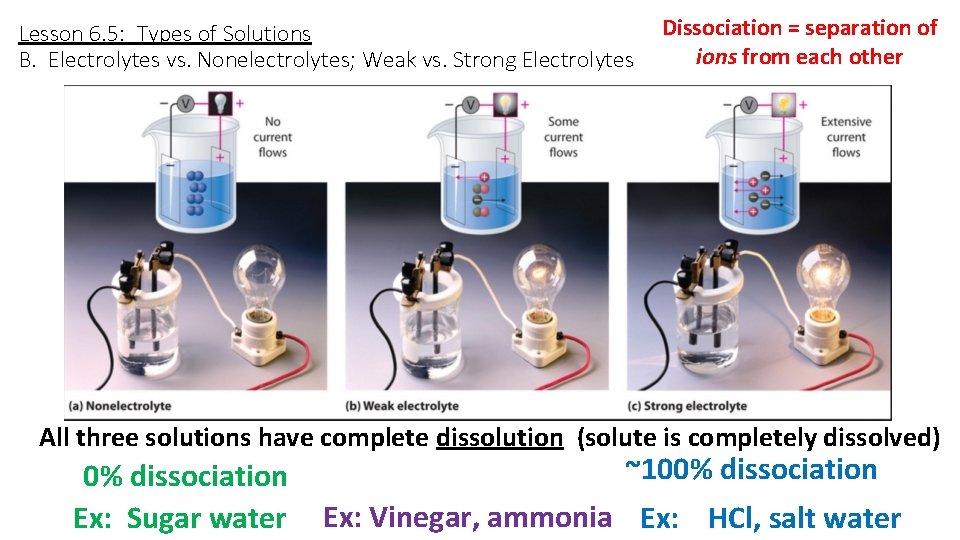
Lesson 6. 5: Types of Solutions B. Electrolytes vs. Nonelectrolytes; Weak vs. Strong Electrolytes Dissociation = separation of ions from each other All three solutions have complete dissolution (solute is completely dissolved) 0% dissociation Ex: Sugar water ~100% dissociation Ex: Vinegar, ammonia Ex: HCl, salt water

DEMO and VIDEO • Water, Gatorade, Monster • Any of you have drinks you want to test? (You can’t drink them afterwards, obviously…)

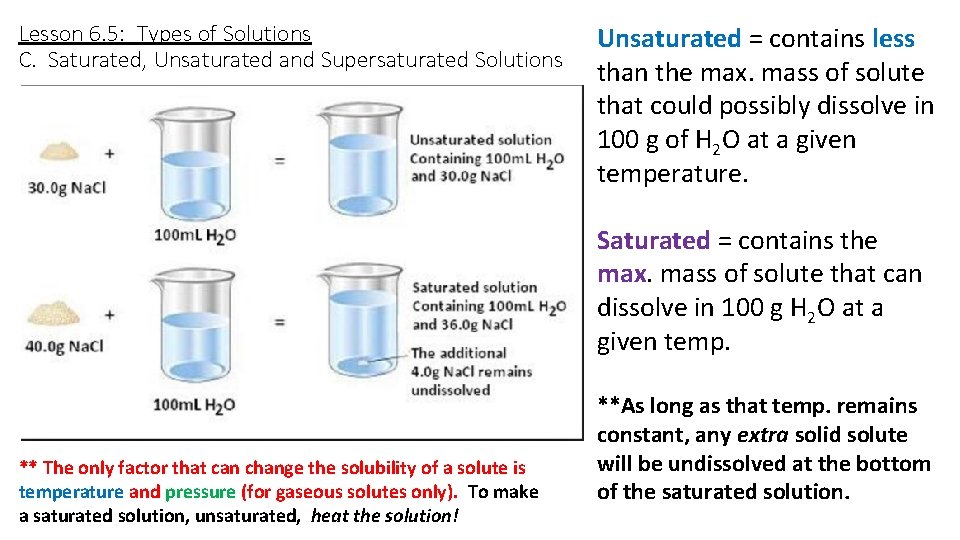
Lesson 6. 5: Types of Solutions C. Saturated, Unsaturated and Supersaturated Solutions Unsaturated = contains less than the max. mass of solute that could possibly dissolve in 100 g of H 2 O at a given temperature. Saturated = contains the max. mass of solute that can dissolve in 100 g H 2 O at a given temp. ** The only factor that can change the solubility of a solute is temperature and pressure (for gaseous solutes only). To make a saturated solution, unsaturated, heat the solution! **As long as that temp. remains constant, any extra solid solute will be undissolved at the bottom of the saturated solution.

VIDEO • Unsaturated, Saturated and Supersaturated Project

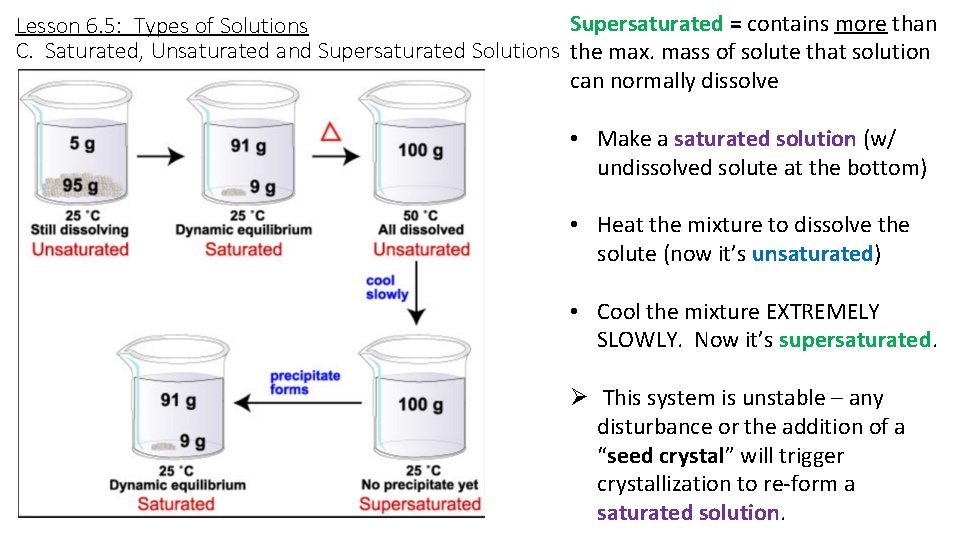
Supersaturated = contains more than Lesson 6. 5: Types of Solutions C. Saturated, Unsaturated and Supersaturated Solutions the max. mass of solute that solution can normally dissolve • Make a saturated solution (w/ undissolved solute at the bottom) • Heat the mixture to dissolve the solute (now it’s unsaturated) • Cool the mixture EXTREMELY SLOWLY. Now it’s supersaturated. Ø This system is unstable – any disturbance or the addition of a “seed crystal” will trigger crystallization to re-form a saturated solution.

QUICK LAB and VIDEO • Crystallization • There are three setups. • Perform a test to see if the two test tubes contain an unsaturated, saturated or supersaturated solution. • Obtain a test tube that contains a warmed solution. Perform a test to see if this test tube contains an unsaturated, saturated or supersaturated solution. Return this test tube to the hot water bath.

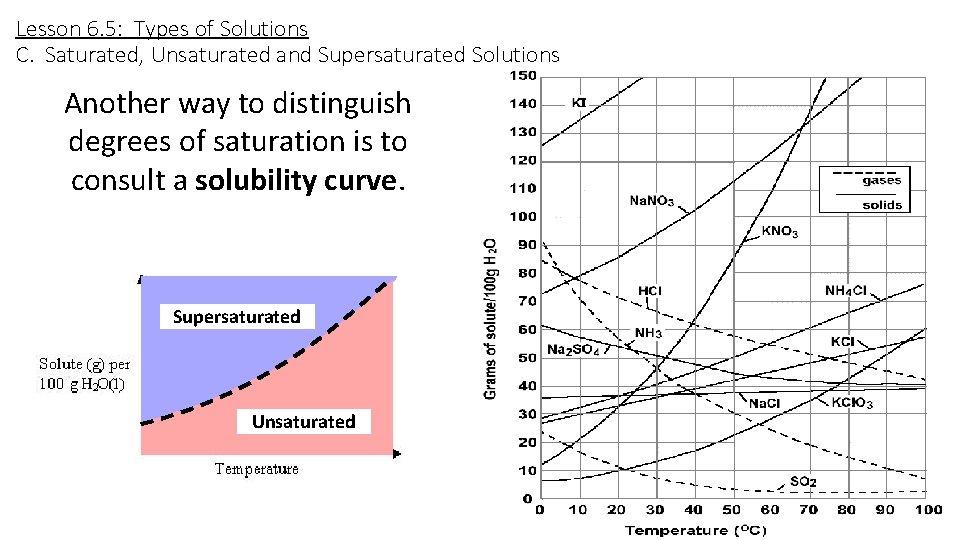
Lesson 6. 5: Types of Solutions C. Saturated, Unsaturated and Supersaturated Solutions Another way to distinguish degrees of saturation is to consult a solubility curve. Supersaturated Unsaturated

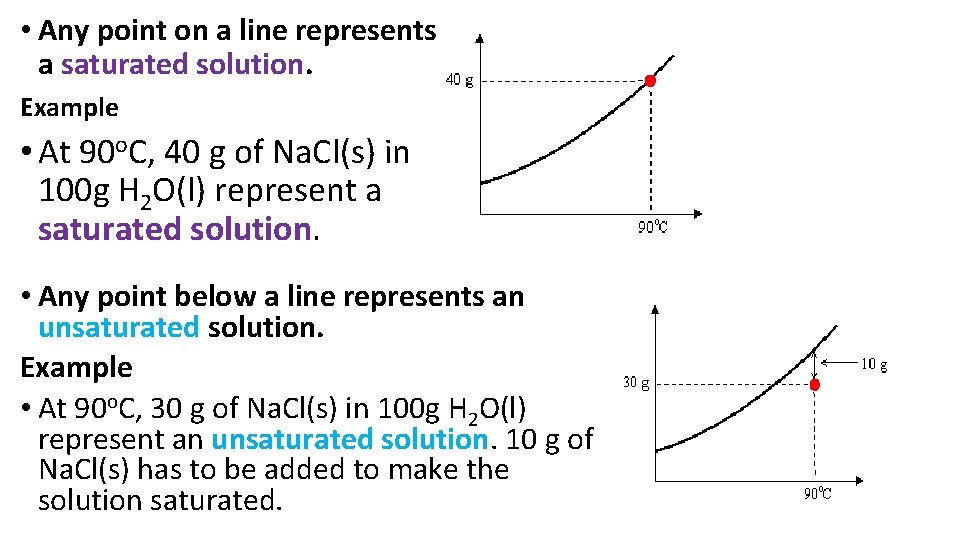
• Any point on a line represents a saturated solution. Example • At 90 o. C, 40 g of Na. Cl(s) in 100 g H 2 O(l) represent a saturated solution. • Any point below a line represents an unsaturated solution. Example • At 90 o. C, 30 g of Na. Cl(s) in 100 g H 2 O(l) represent an unsaturated solution. 10 g of Na. Cl(s) has to be added to make the solution saturated.

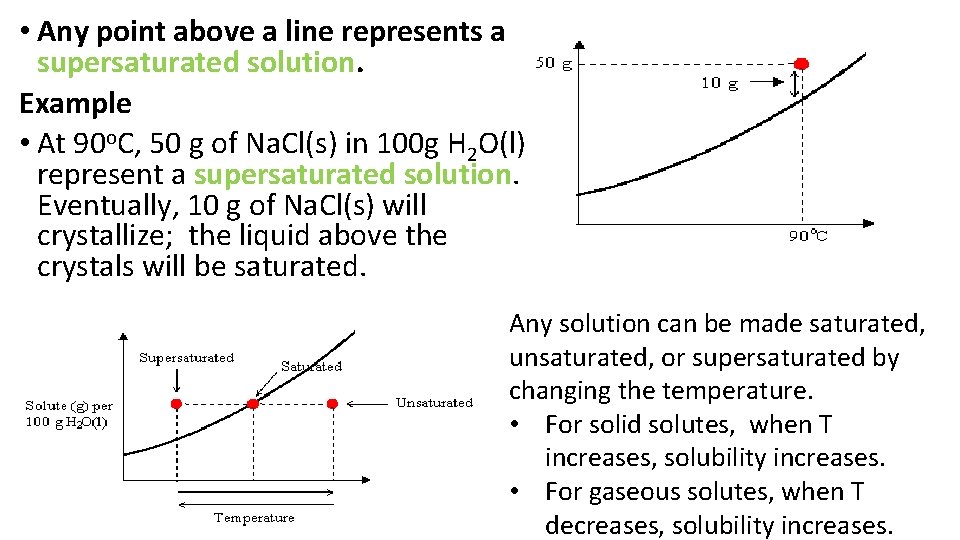
• Any point above a line represents a supersaturated solution. Example • At 90 o. C, 50 g of Na. Cl(s) in 100 g H 2 O(l) represent a supersaturated solution. Eventually, 10 g of Na. Cl(s) will crystallize; the liquid above the crystals will be saturated. Any solution can be made saturated, unsaturated, or supersaturated by changing the temperature. • For solid solutes, when T increases, solubility increases. • For gaseous solutes, when T decreases, solubility increases.


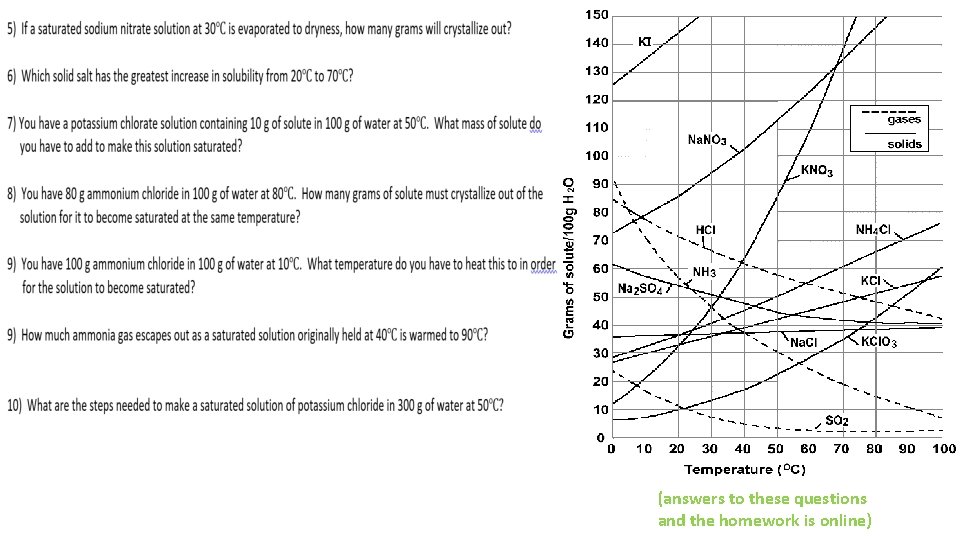
(answers to these questions and the homework is online)

Let’s go over the test…