Sample Size for Phase II Trials Simons Design












- Slides: 28

Sample Size for Phase II Trials: Simon's Design and MCP-Mod Case Studies

§ Head of Statistics § n. Query Lead Researcher § FDA Guest Speaker § Guest Lecturer HOSTED BY: Ronan Fitzpatrick

Overview Phase II Designs & SSD Simon’s Design MCP-Mod Discussion & Conclusions

Worked Examples Overview WORKED EXAMPLES üSimon’s Design üSingle and Three-stage designs üMCP-Mod (continuous outcome)

About n. Query In 2018, 91% of organizations with clinical trials approved by the FDA used n. Query for sample size and power calculation

PAR T 1 Phase II Trials & SSD

Clinical Trials Phases 3 pre-approval clinical trial phases: I, II (IIa/IIb), III Phase IV: Post-approval evaluation Phase I: First-in-human in trial (10 -50 subjects) Evaluate safety in healthy volunteers (find MTD) Phase II: Proof-of-concept & Dose-Finding (50 -1000) Often split POC & DF into two distinct phases of IIa and IIb Phase III: Confirmatory trial (300 -5000 subjects) Prove efficacy and safety of treatment for regulatory approval

SSD in Phase II SSD finds the appropriate sample size for your study Balance ethical & practical: Validity, cost, regulatory etc. Usually associated with Phase III but what about Phase II? Need SSD methods for Phase II designs and statistical methods Phase II has high failure rate so adaptive designs useful Phase IIa: Emphasise quick evaluation, early futility

Phase IIa & IIb Phase IIa: Proof-of-Concept Trial (50 -100 subjects) Find proof of positive response for proposed treatment to recommend for further clinical trial evaluation Example Methods: One sample, Fleming Test, Simon’s Design Phase IIb: Dose-Finding (100 -100 subjects) Find dose-response curve to see dose-response relationship, evaluate if strong enough response & if so select Phase III doses Examples Methods: Contrast tests, Cochran-Armitage, MCP-Mod

PAR T 2 Simon’s Design

Phase IIa Methods Proof-of-concept trial focused on if “response” exists Response often yes/no response evaluation (e. g. tumour regression) Want to quickly evaluate if response of interest exists Early rejection for futility useful as high failure rate expected Several tests developed to allow this choice at interim Examples: Gehan (1961), Fleming (1982), Bryant & Day (1995) One of the most popular is Simon’s 2 -stage design

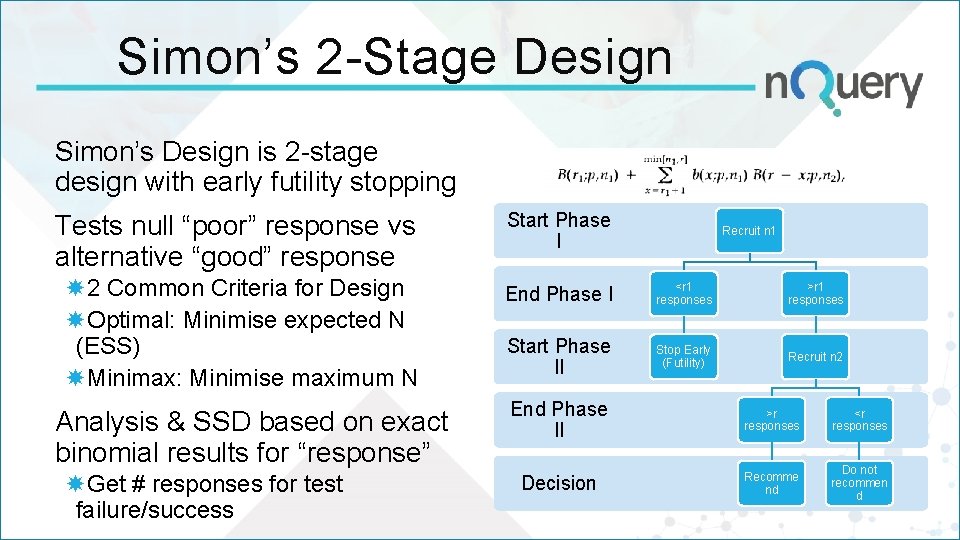
Simon’s 2 -Stage Design Simon’s Design is 2 -stage design with early futility stopping Tests null “poor” response vs alternative “good” response Start Phase I 2 Common Criteria for Design Optimal: Minimise expected N (ESS) Minimax: Minimise maximum N End Phase I <r 1 responses >r 1 responses Start Phase II Stop Early (Futility) Recruit n 2 Analysis & SSD based on exact binomial results for “response” Get # responses for test failure/success Recruit n 1 End Phase II >r responses <r responses Decision Recomme nd Do not recommen d

p le m a Ex 1 “The primary end point of this study was the MRD response rate, as defined by the incidence of MRD negativity within four cycles of treatment with blinatumomab. Simon’s Min. Max two-stage design with parameters P 0 = 0. 05, P 1 = 0. 3, α = 0. 05, and β = 0. 2 resulted in two stages of seven patients each. “ Parameter Value Test Significance Level (α) 0. 05 “Poor” Response (π1) 0. 05 “Good” Response (π1) 0. 3 Power 80 Source: Journal of Clinical Oncology (2011)

Simon’s Design Notes Optimal usually recommended unless expected N close to minimax or expect slow accrual time Minimax has larger first phase and higher E(N) n. Query default method not g’teed to find global minimum Starts search at minimum of single-stage design or fixed term N However, trade-off for speed unlikely to require starting from 2 Method can be extended to 3 -phases (or further) Rapidly increases complexity; 3 -stage: POT 12 in n. Query Many other versions proposed, recently adaptive versions popular

PAR T 3 MCP-Mod

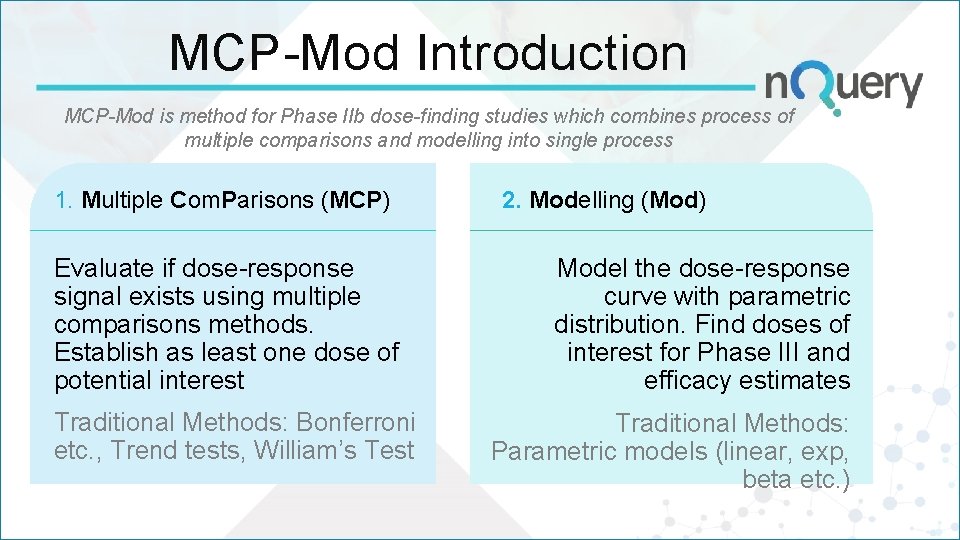
MCP-Mod Introduction MCP-Mod is method for Phase IIb dose-finding studies which combines process of multiple comparisons and modelling into single process 1. Multiple Com. Parisons (MCP) Evaluate if dose-response signal exists using multiple comparisons methods. Establish as least one dose of potential interest Traditional Methods: Bonferroni etc. , Trend tests, William’s Test 2. Modelling (Mod) Model the dose-response curve with parametric distribution. Find doses of interest for Phase III and efficacy estimates Traditional Methods: Parametric models (linear, exp, beta etc. )

MCP-Mod Justification MCP & modelling typically done separately/one done • MCP: Robust but restricted to selected doses as nominal variable • Mod: Flexible quantitative approach but reliant on model choice MCP-Mod combines both into single method (Bretz et al) • Design Stage: Select candidate models & generate optimal tests • MCP Stage: Select best model(s) w/ appropriate contrasts but control FWER • Mod Stage: Model using best model and find target dose(s) of interest EMA CHMP (2014) & FDA (2016): “fit-for-purpose”

Source: Dose. Finding (CRAN) Source: Bretz et al (2005), Pinhiero et al. (2014)

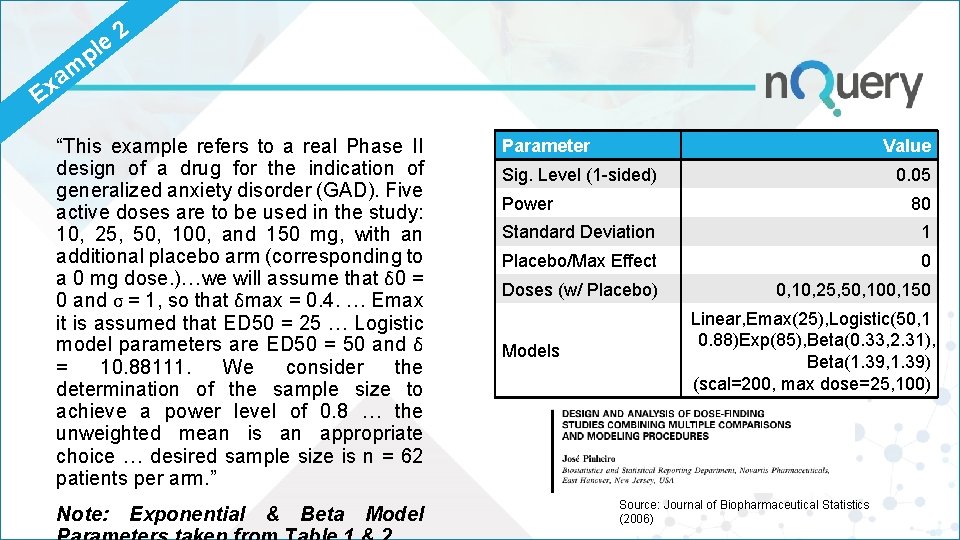
p le m a Ex 2 “This example refers to a real Phase II design of a drug for the indication of generalized anxiety disorder (GAD). Five active doses are to be used in the study: 10, 25, 50, 100, and 150 mg, with an additional placebo arm (corresponding to a 0 mg dose. )…we will assume that δ 0 = 0 and σ = 1, so that δmax = 0. 4. … Emax it is assumed that ED 50 = 25 … Logistic model parameters are ED 50 = 50 and δ = 10. 88111. We consider the determination of the sample size to achieve a power level of 0. 8 … the unweighted mean is an appropriate choice … desired sample size is n = 62 patients per arm. ” Note: Exponential & Beta Model Parameter Value Sig. Level (1 -sided) 0. 05 Power 80 Standard Deviation 1 Placebo/Max Effect 0 Doses (w/ Placebo) 0, 10, 25, 50, 100, 150 Models Linear, Emax(25), Logistic(50, 1 0. 88)Exp(85), Beta(0. 33, 2. 31), Beta(1. 39, 1. 39) (scal=200, max dose=25, 100) Source: Journal of Biopharmaceutical Statistics (2006)

MCP-Mod Notes Flexible to # doses, # models, outcome (continuous, binary, TTE) • 4 -7 doses typical, 8 models in n. Query, control dose typically included Multiple models may be considered at “Mod” stage MCP selection exclusively related to contrast p-values by default Method has been extended to other types of test MCLRa, MCLRe: Likelihood ratio rather than MCP contrast test MHMC: Hierarchial Sequential Test (selects and estimates model)

PAR T 4 Discussion and Conclusions

Discussion and Conclusions Phase II trials for proof-of-concept & pick Phase III doses Phase IIa: Proof-of-concept; Phase IIb: Dose-finding SSD methods desirable for common methods for both Phase IIa methods like Simon’s Design looking for evidence of “response” and will stop early if no signal. Phase IIb changed significantly by combining robust MCP with modelling rather than separating these objectives Sample size methods for Phase II methods allows wider usage & better outcomes at high failure clinical

n. Query Winter 2019 Update Overhauls the workflow and plotting functionality of n. Query while adding new tables for classical, Bayesian and adaptive designs www. statsols. com/whats-new 2 New Software Improvements Improved Fully Editable Plots Simplified Welcome Screen 16 New Classical & Bayesian Tables Count Models One Proportion Credible Intervals Survival NI 6 New Adaptive Design Tables Counts GST Phase II Designs MCP-Mod


Q&A Any Questions? For further details, contact at: info@statsols. com Thanks for listening!

References De. Mets, D. , Friedman, L. , and Furberg, C. (2010). Fundamentals of Clinical Trials (4 th ed. ). Springer. Ting, N; Chen, DG; Ho, S; Cappelleri, J; (2017). Phase II Clinical Development of New Drugs. Springer. Fleming, T. R. , 1982. One-sample multiple testing procedure for phase II clinical trials. Biometrics, pp. 143 -151. Gehan, E. A. , 1961. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. Journal of chronic diseases, 13(4), pp. 346353. Bryant, J. and Day, R. , 1995. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics, pp. 13721383.

References Simon, R. , 1989. Optimal two-stage designs for phase II clinical trials. Controlled clinical trials, 10(1), pp. 1 -10. Jung, S. H. , Lee, T. , Kim, K. and George, S. L. , 2004. Admissible two‐stage designs for phase II cancer clinical. Statistics trials. in medicine, 23(4), pp. 561 -569. Topp, M. S. , Kufer, P. , Gökbuget, N. , Goebeler, M. , Klinger, M. , Neumann, S. , Horst, H. A. , Raff, T. , Viardot, A. , Schmid, M. and Stelljes, M. , 2011. Targeted therapy with the T-cell–engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. Journal of clinical oncology, 29(18), pp. 2493 -2498. Englert, S. and Kieser, M. , 2013. Optimal adaptive two‐stage designs for phase II cancer clinical trials. Biometrical Journal, 55(6), pp. 955 -968.

References Bretz, F. , Pinheiro, J. C. and Branson, M. , 2005. Combining multiple comparisons and modeling techniques in dose‐response studies. Biometrics, 61(3), pp. 738 -748. Pinheiro, J. , Bornkamp, B. and Bretz, F. , 2006. Design and analysis of dose-finding studies combining multiple comparisons and modeling procedures. Journal of biopharmaceutical statistics, 16(5), pp. 639 -656. Bornkamp, B. , Bretz, F. , Dmitrienko, A. , Enas, G. , Gaydos, B. , Hsu, C. H. , König, F. , Krams, M. , Liu, Q. , Neuenschwander, B. and Parke, T. , 2007. Innovative approaches for designing and analyzing adaptive dose-ranging trials. Journal of biopharmaceutical statistics, 17(6), pp. 965 -995. Ruberg, S. J. , 1995. Dose response studies I. Some design considerations. Journal of Biopharmaceutical Statistics, 5(1), pp. 1 -14.