Salt Effects Lecture 5 Yuri Kazakevich Seton Hall


![A Concentration Versus p. H ? Total Cl. O 4[m. M] B 47 41 A Concentration Versus p. H ? Total Cl. O 4[m. M] B 47 41](https://slidetodoc.com/presentation_image_h/dc7502998671e5ea3d04ce3e22142495/image-13.jpg)
- Slides: 30

Salt Effects Lecture 5 Yuri Kazakevich Seton Hall University 1

Effect of Modifier Type and Concentration of Salt on HPLC Separations • Analyte Solvation • Retention of Basic compounds in low p. H region • Increase in retention • Concentration vs. p. H? • Chaotropic effect • Disruption of solvation • Effect of counteranion concentration • Type of counteranion 2

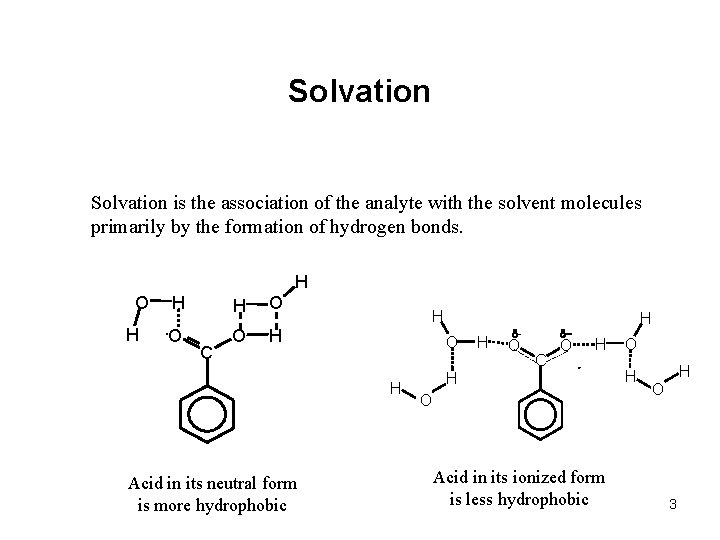
Solvation is the association of the analyte with the solvent molecules primarily by the formation of hydrogen bonds. H O H H H O O O H C H O H Acid in its neutral form is more hydrophobic H H d- d- O C O H H O Acid in its ionized form is less hydrophobic O H H O 3

Solvation with Eluent Components CH 3 CN/H 2 O CH 3 OH/H 2 O H H O H d- d- O C O H H d- d- O • Acetonitrile is not able to solvate analyte since it cannot form hydrogen bonds. • Solvation with methanol forms a partially hydrophilic shell that could be retained on the RP adsorbent. 4

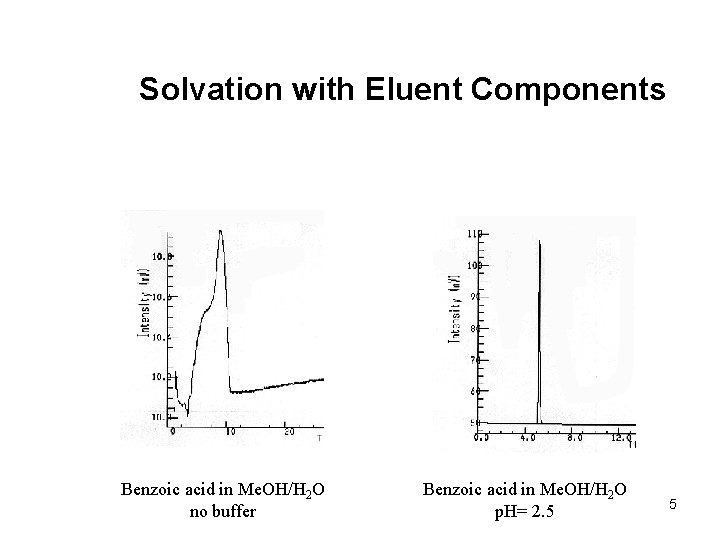
Solvation with Eluent Components Benzoic acid in Me. OH/H 2 O no buffer Benzoic acid in Me. OH/H 2 O p. H= 2. 5 5

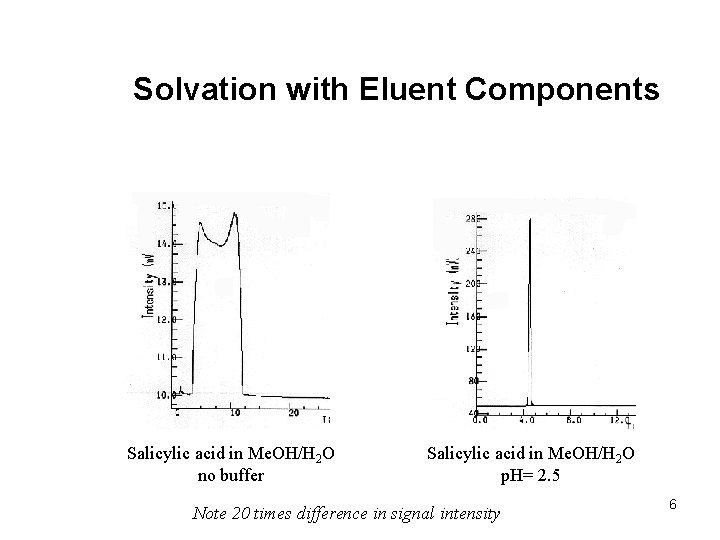
Solvation with Eluent Components Salicylic acid in Me. OH/H 2 O no buffer Salicylic acid in Me. OH/H 2 O p. H= 2. 5 Note 20 times difference in signal intensity 6

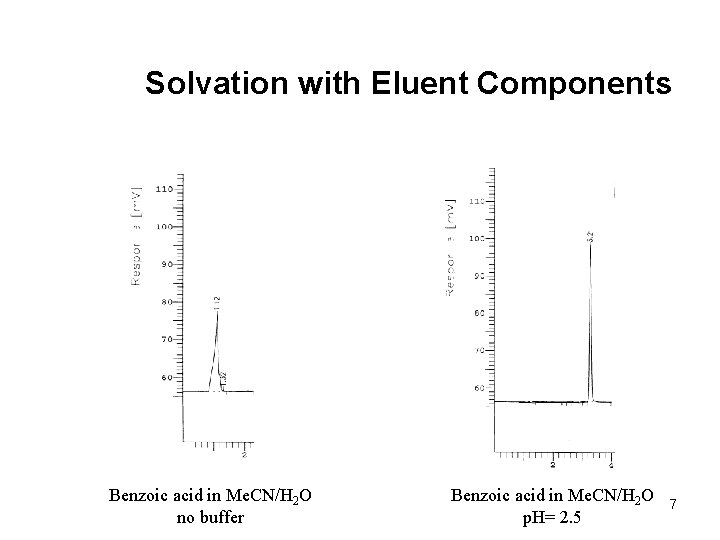
Solvation with Eluent Components Benzoic acid in Me. CN/H 2 O no buffer Benzoic acid in Me. CN/H 2 O p. H= 2. 5 7

Eluent Additives Buffer components: Salt, Acid Retention of Basic Compounds in a Low p. H Region is Affected by Salt Concentration and Type of Acid • • • Basic compounds that are fully ionized have a low retention. Goal is to increase basic analyte retention in a low p. H region. The addition of various acids and salts to the mobile phase may effect the retention of protonated basic analytes. 8

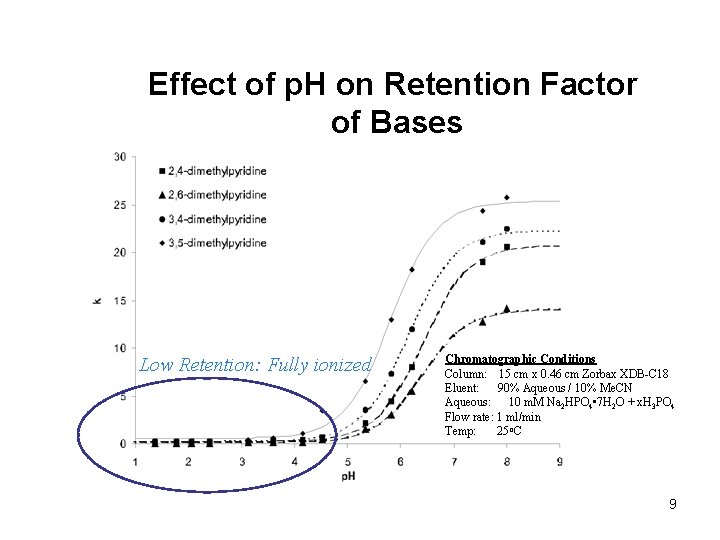
Effect of p. H on Retention Factor of Bases Low Retention: Fully ionized Chromatographic Conditions Column: 15 cm x 0. 46 cm Zorbax XDB-C 18 Eluent: 90% Aqueous / 10% Me. CN Aqueous: 10 m. M Na 2 HPO 4 • 7 H 2 O + x. H 3 PO 4 Flow rate: 1 ml/min Temp: 25 o. C 9

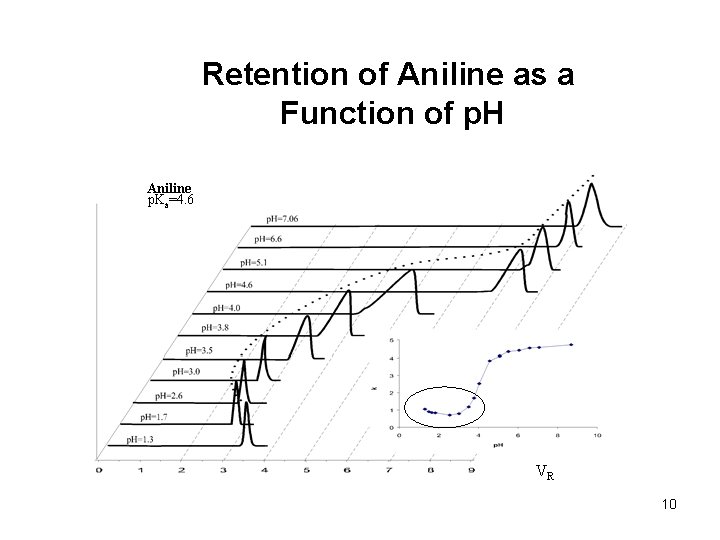
Retention of Aniline as a Function of p. H Aniline p. Ka=4. 6 VR 10

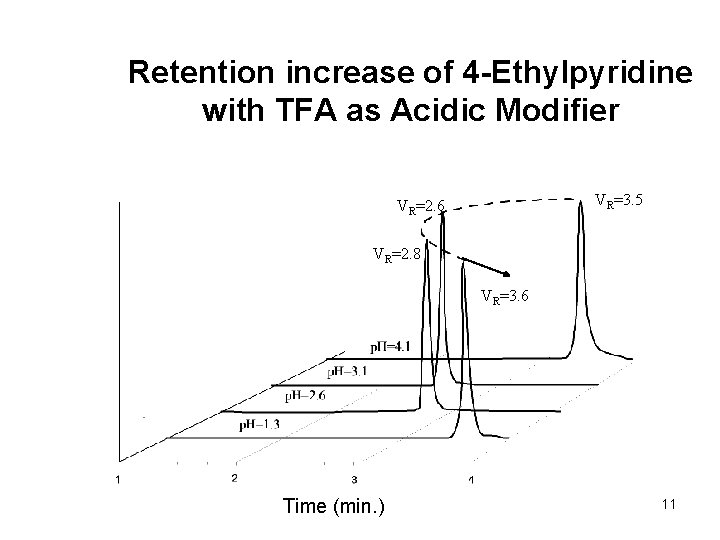
Retention increase of 4 -Ethylpyridine with TFA as Acidic Modifier VR=3. 5 VR=2. 6 VR=2. 8 VR=3. 6 Time (min. ) 11

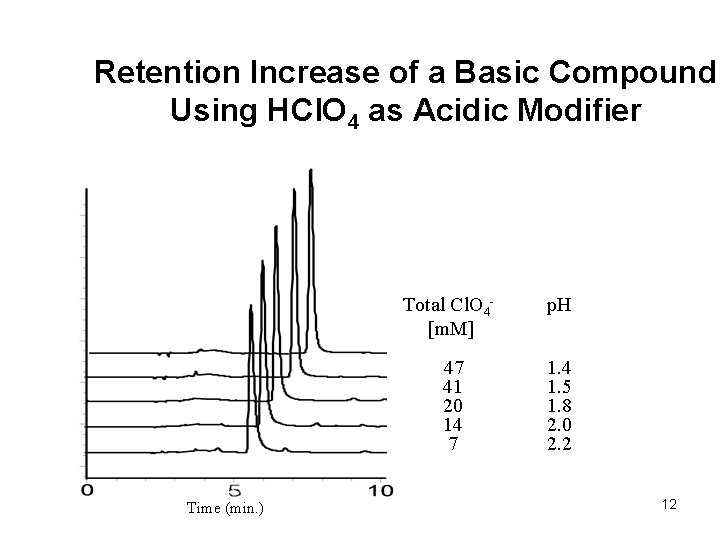
Retention Increase of a Basic Compound Using HCl. O 4 as Acidic Modifier Time (min. ) Total Cl. O 4[m. M] p. H 47 41 20 14 7 1. 4 1. 5 1. 8 2. 0 2. 2 12
![A Concentration Versus p H Total Cl O 4m M B 47 41 A Concentration Versus p. H ? Total Cl. O 4[m. M] B 47 41](https://slidetodoc.com/presentation_image_h/dc7502998671e5ea3d04ce3e22142495/image-13.jpg)
A Concentration Versus p. H ? Total Cl. O 4[m. M] B 47 41 20 14 7 p. H 1. 4 1. 5 1. 8 2. 0 2. 2 Total Cl. O 4[m. M] 100 89 79 70 55 Rt. (min. ) 7. 5 5. 2 p. H 2. 0 2. 0 Rt. (min. ) 9. 9 7. 7 13

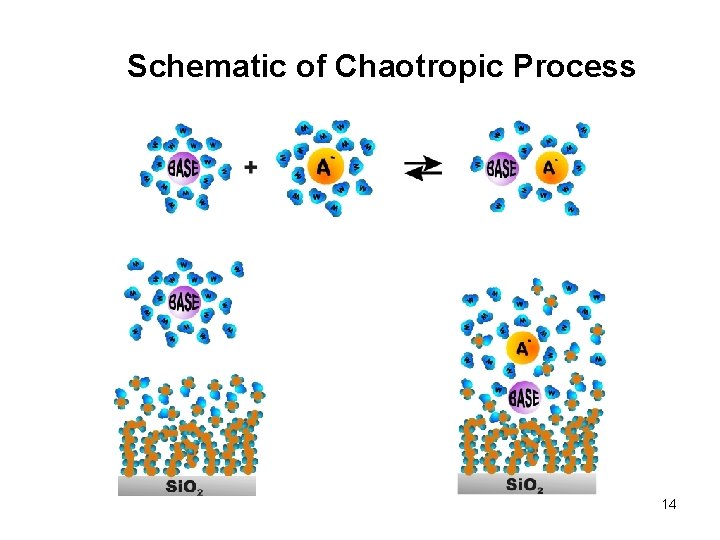
Schematic of Chaotropic Process 14

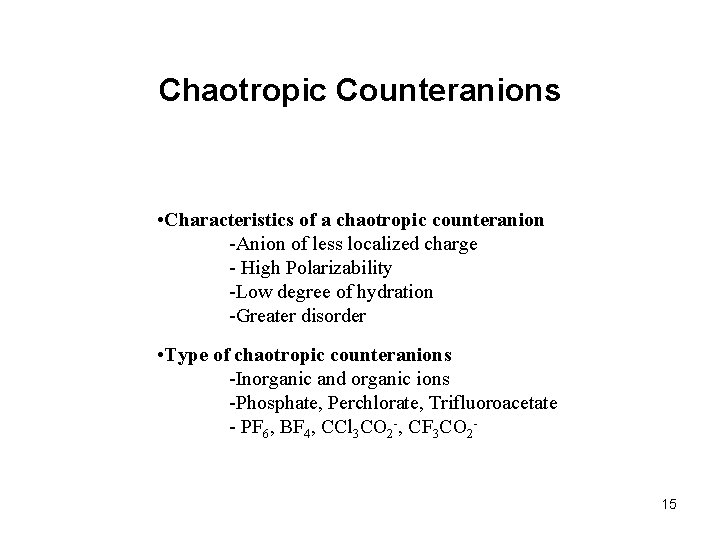
Chaotropic Counteranions • Characteristics of a chaotropic counteranion -Anion of less localized charge - High Polarizability -Low degree of hydration -Greater disorder • Type of chaotropic counteranions -Inorganic and organic ions -Phosphate, Perchlorate, Trifluoroacetate - PF 6, BF 4, CCl 3 CO 2 -, CF 3 CO 2 - 15

Anionic Chaotropes in Reversed Phase HPLC • Basic analyte must be fully ionized in order to ensure electrostatic interaction with anionic chaotrope. • Effects retention of Basic Analytes. • Hydrogen bonding between water molecules disrupted. • Decrease in solvation of protonated basic analyte since hydration shield around protonated analyte becomes less structured. • Facilitate the approach and increased interaction of the analyte to the stationary phase. • Retention generally increases with increase in counteranion concentration. • Changes in selectivity may be observed. 16

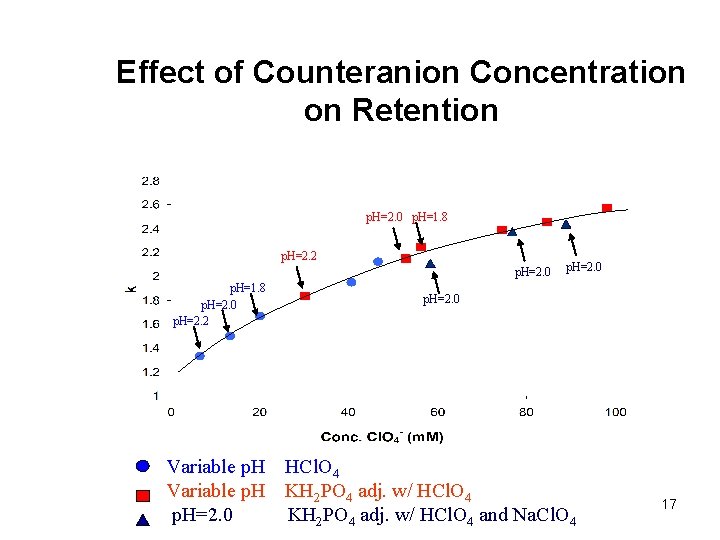
Effect of Counteranion Concentration on Retention p. H=2. 0 p. H=1. 8 p. H=2. 2 p. H=2. 0 p. H=1. 8 p. H=2. 0 p. H=2. 2 Variable p. H=2. 0 p. H=2. 0 HCl. O 4 KH 2 PO 4 adj. w/ HCl. O 4 and Na. Cl. O 4 17

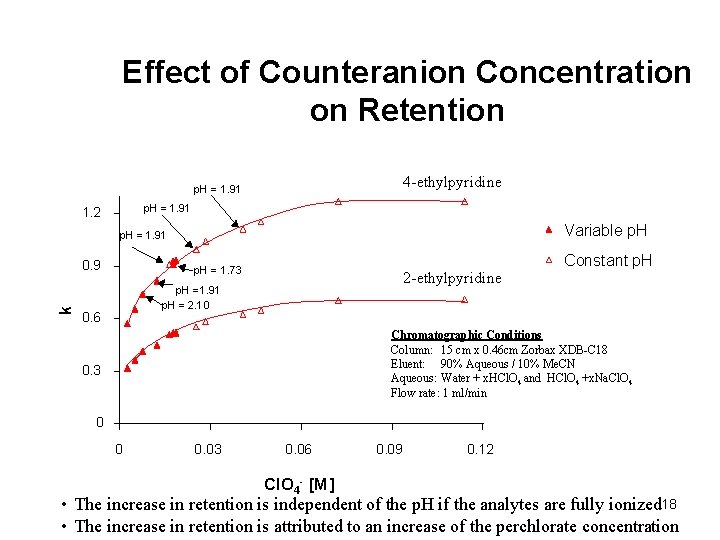
Effect of Counteranion Concentration on Retention 4 -ethylpyridine p. H = 1. 91 1. 2 Variable p. H = 1. 91 k 0. 9 p. H = 1. 73 2 -ethylpyridine p. H =1. 91 p. H = 2. 10 0. 6 Constant p. H Chromatographic Conditions Column: 15 cm x 0. 46 cm Zorbax XDB-C 18 Eluent: 90% Aqueous / 10% Me. CN Aqueous: Water + x. HCl. O 4 and HCl. O 4 +x. Na. Cl. O 4 Flow rate: 1 ml/min 0. 3 0 0 0. 03 0. 06 Cl. O 4 - [M] 0. 09 0. 12 • The increase in retention is independent of the p. H if the analytes are fully ionized 18 • The increase in retention is attributed to an increase of the perchlorate concentration

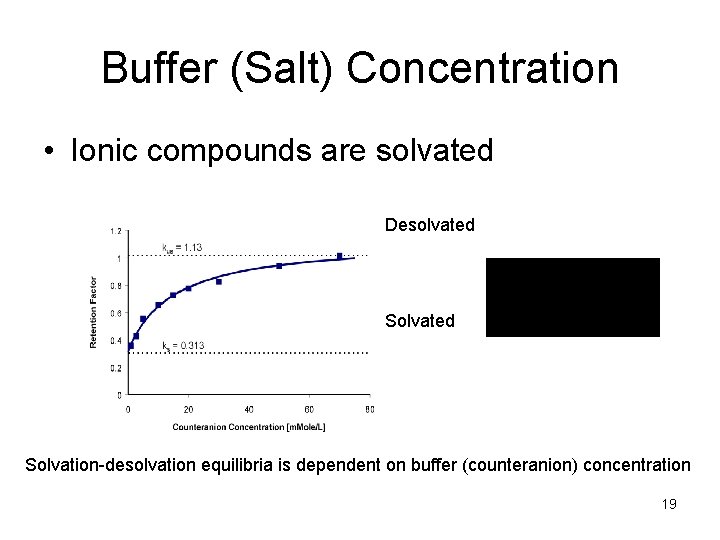
Buffer (Salt) Concentration • Ionic compounds are solvated Desolvated Solvation-desolvation equilibria is dependent on buffer (counteranion) concentration 19

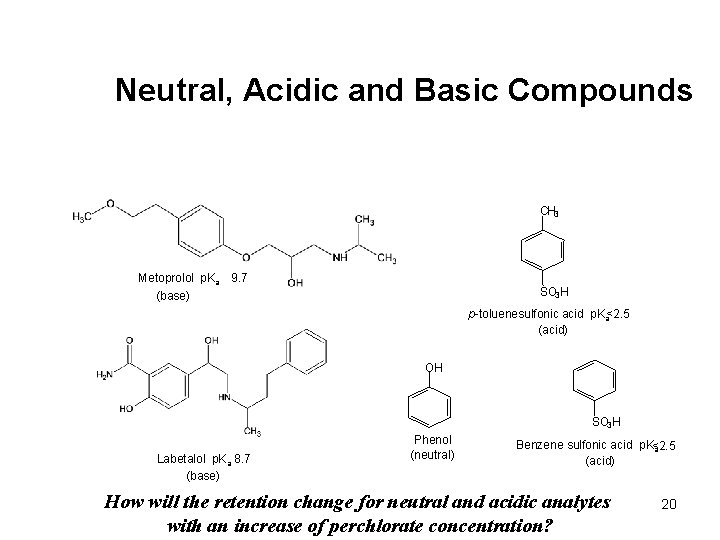
Neutral, Acidic and Basic Compounds CH 3 Metoprolol p. Ka 9. 7 (base) SO 3 H p-toluenesulfonic acid p. Ka<2. 5 (acid) OH SO 3 H Labetalol p. Ka 8. 7 (base) Phenol (neutral) Benzene sulfonic acid p. K<2. 5 a (acid) How will the retention change for neutral and acidic analytes with an increase of perchlorate concentration? 20

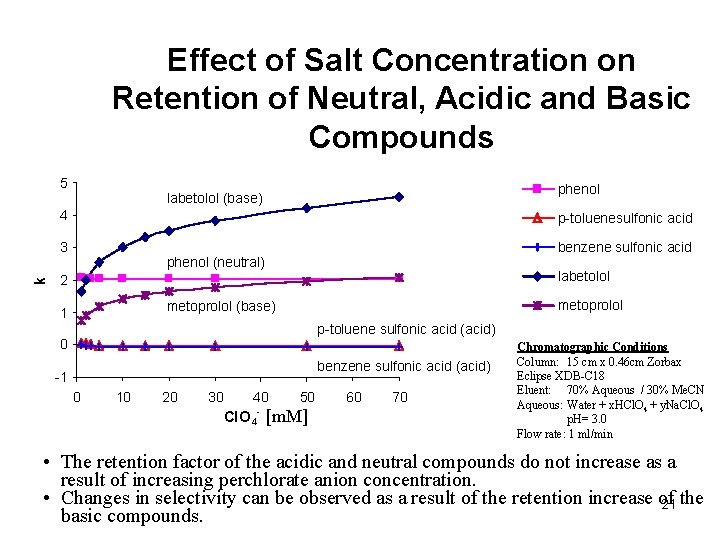
Effect of Salt Concentration on Retention of Neutral, Acidic and Basic Compounds k 5 phenol labetolol (base) 4 p-toluenesulfonic acid 3 benzene sulfonic acid phenol (neutral) labetolol 2 metoprolol (base) 1 p-toluene sulfonic acid (acid) 0 benzene sulfonic acid (acid) -1 0 10 20 30 40 Cl. O 4 - 50 [m. M] 60 70 Chromatographic Conditions Column: 15 cm x 0. 46 cm Zorbax Eclipse XDB-C 18 Eluent: 70% Aqueous / 30% Me. CN Aqueous: Water + x. HCl. O 4 + y. Na. Cl. O 4 p. H= 3. 0 Flow rate: 1 ml/min • The retention factor of the acidic and neutral compounds do not increase as a result of increasing perchlorate anion concentration. • Changes in selectivity can be observed as a result of the retention increase of 21 the basic compounds.

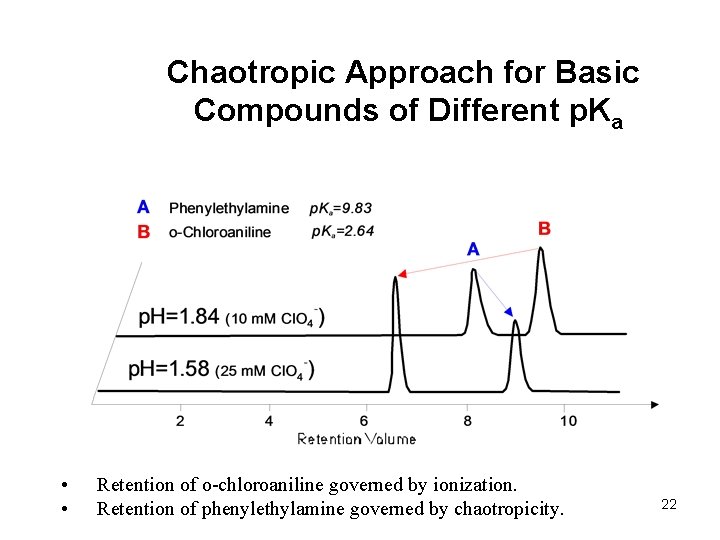
Chaotropic Approach for Basic Compounds of Different p. Ka • • Retention of o-chloroaniline governed by ionization. Retention of phenylethylamine governed by chaotropicity. 22

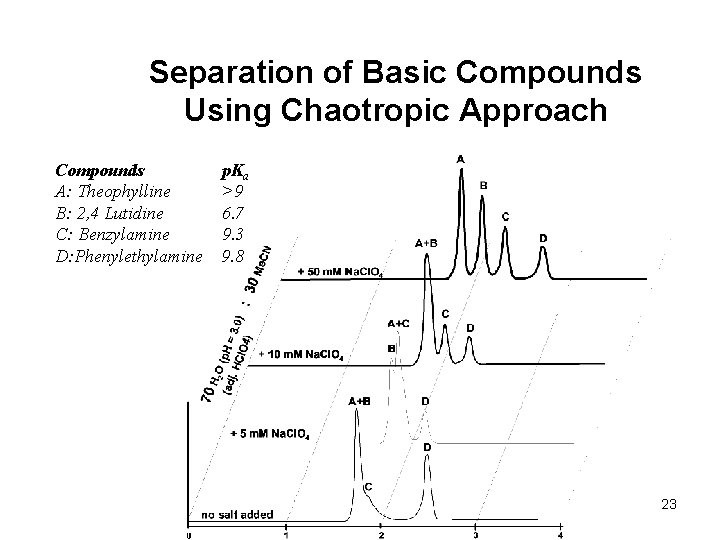
Separation of Basic Compounds Using Chaotropic Approach Compounds A: Theophylline B: 2, 4 Lutidine C: Benzylamine D: Phenylethylamine p. Ka >9 6. 7 9. 3 9. 8 23

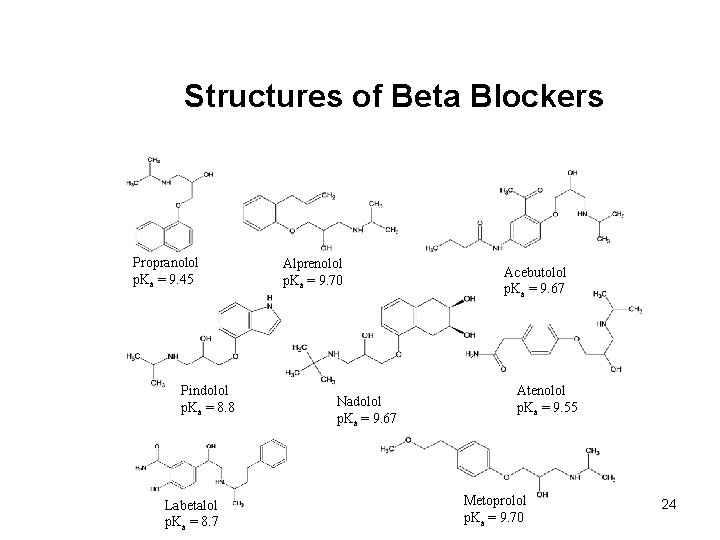
Structures of Beta Blockers Propranolol p. Ka = 9. 45 Pindolol p. Ka = 8. 8 Labetalol p. Ka = 8. 7 Alprenolol p. Ka = 9. 70 Nadolol p. Ka = 9. 67 Acebutolol p. Ka = 9. 67 Atenolol p. Ka = 9. 55 Metoprolol p. Ka = 9. 70 24

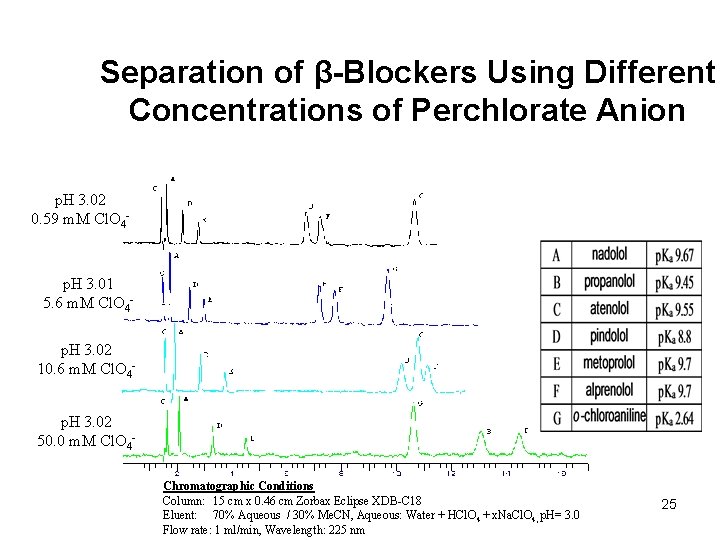
Separation of β-Blockers Using Different Concentrations of Perchlorate Anion p. H 3. 02 0. 59 m. M Cl. O 4 - p. H 3. 01 5. 6 m. M Cl. O 4 p. H 3. 02 10. 6 m. M Cl. O 4 p. H 3. 02 50. 0 m. M Cl. O 4 Chromatographic Conditions Column: 15 cm x 0. 46 cm Zorbax Eclipse XDB-C 18 Eluent: 70% Aqueous / 30% Me. CN, Aqueous: Water + HCl. O 4 + x. Na. Cl. O 4, p. H= 3. 0 Flow rate: 1 ml/min, Wavelength: 225 nm 25

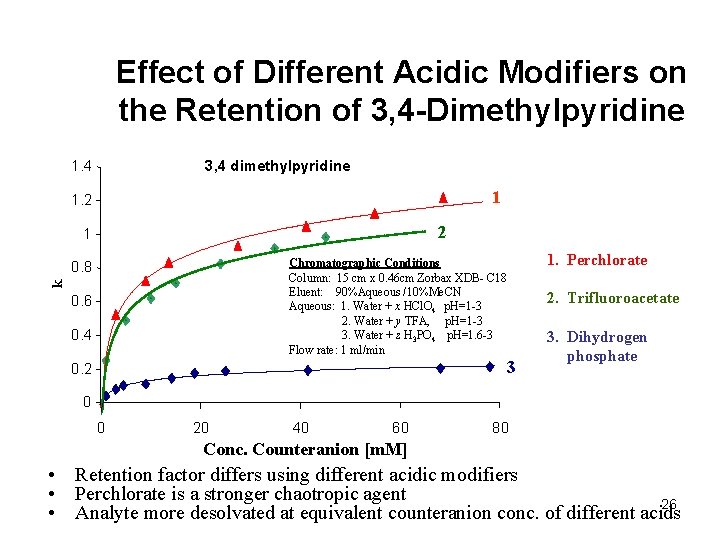
Effect of Different Acidic Modifiers on the Retention of 3, 4 -Dimethylpyridine 3, 4 dimethylpyridine 1. 4 1 1. 2 2 1 Chromatographic Conditions Column: 15 cm x 0. 46 cm Zorbax XDB- C 18 Eluent: 90%Aqueous /10%Me. CN Aqueous: 1. Water + x HCl. O 4 p. H=1 -3 2. Water + y TFA, p. H=1 -3 3. Water + z H 3 PO 4 p. H=1. 6 -3 Flow rate: 1 ml/min k 0. 8 0. 6 0. 4 3 0. 2 1. Perchlorate 2. Trifluoroacetate 3. Dihydrogen phosphate 0 0 20 40 60 80 Conc. Counteranion [m. M] • Retention factor differs using different acidic modifiers • Perchlorate is a stronger chaotropic agent 26 • Analyte more desolvated at equivalent counteranion conc. of different acids

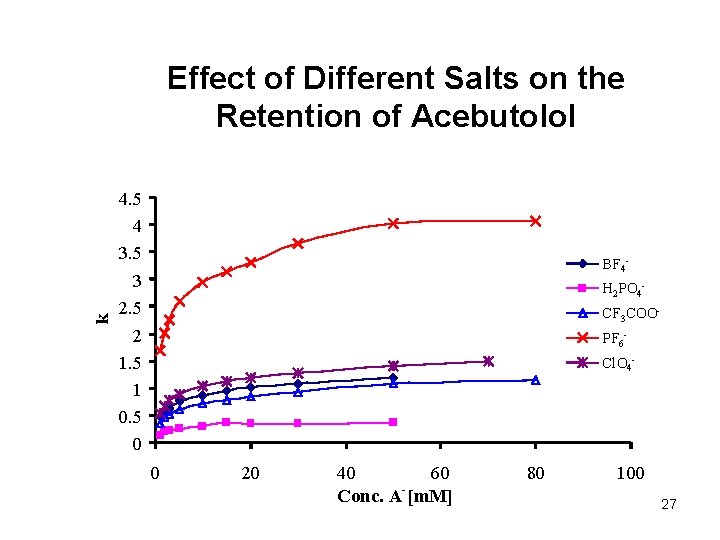
k Effect of Different Salts on the Retention of Acebutolol 4. 5 4 3. 5 3 2. 5 2 1. 5 1 0. 5 0 BF 4 H 2 PO 4 CF 3 COOPF 6 Cl. O 4 - 0 20 40 60 Conc. A [m. M] 80 100 27

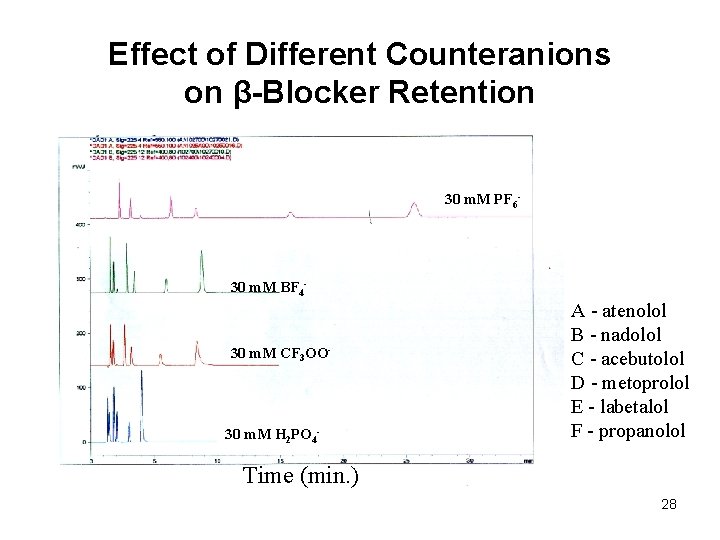
Effect of Different Counteranions on β-Blocker Retention 30 m. M PF 6 A A B C D F D D 30 m. M CF 3 OO- A+ C B D E E F F 30 m. M BF 4 - E A C B E F 30 m. M H 2 PO 4 - A - atenolol B - nadolol C - acebutolol D - metoprolol E - labetalol F - propanolol Time (min. ) 28

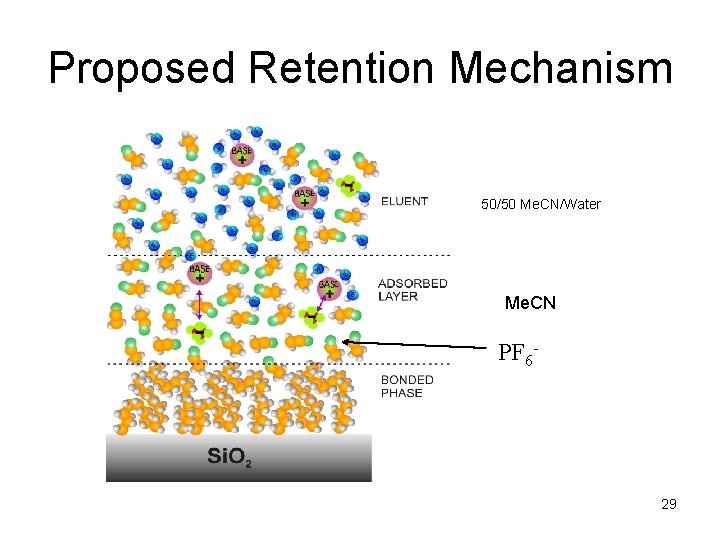
Proposed Retention Mechanism 50/50 Me. CN/Water Me. CN PF 6 - 29

Conclusion The type and concentration of chaotropic counteranions in the mobile phase can increase the retention of protonated basic analytes by disruption of the analyte solvation shell and increase the analyte hydrophobicity. A basic compound must be protonated in order for ion association with the chaotropic counteranion to occur. This increase in analyte retention is not a p. H dependent process. The chaotropic approach for use in HPLC method development has been shown to be beneficial for the development of fast and efficient separation methods. Combination of the ionization effect and the chaotropic influence on the analyte retention gives the chromatographer the flexibility for selectivity adjustment in HPLC separations. 30