Salinity is the total amount of dissolved material







- Slides: 20

Salinity is the total amount of dissolved material in grams in one kilogram of sea water (Ideal, hard to measure) On average, there is around 35 gram salt in a kilogram of sea water, 35 g/kg, written as S=35‰ or S=35 ppt, read as “thirty-five parts per thousand” (non-dimensional) Globally, S can go from near 0 (coast) to about 40 ppt (Red Sea), BUT 90% is between 34 -35 ppt Salinity is an aggregate variable. The most abundant ions in sea water: chlorine Cl- 55. 0% sodium Na+ 30. 6% sulphate SO 4++ 7. 7% magnesium Mg++ 3. 7% potassium K+ 1. 1%

Basic properties of salt in sea water 1). The ratio of the more abundant components remain almost constant. (ocean is very well mixed globally over geological time~millions of years, much longer than the time for water to circulate through the oceans~thousands of years) (Major exceptions are found in coastal and enclosed regions, due to land drainage and biological processes) 2). There are significant differences in total concentration of the dissolved salts from place to place and at different depths. (Processes, such as local evaporation and precipitation, as well as oceanic circulation, continually concentrate and dilute salt in sea water in specific localities) 3). The presence of salts influences most physical properties of sea water (density, compressibility, freezing point). 4). The conductivity of the sea water is partly determined by the amount of salt it contains.

Early Definition (1902) Given by a commission of the International Council for the Exploration of the Sea Total amount of solid materials in grams dissolved in one kilogram of sea water when all the carbonate has been converted to oxide, the bromine and iodine replaced by chlorine and all organic matter completely oxidized

Salinity based on chlorinity Salinity can be determined through the amount of chloride ion (plus the chlorine equivalent of the bromine and iodine), called as chlorinity, which is measured using titration with silver nitrate (Knudsen et al. , 1902) The relationship between salinity and chlorinity is based on laboratory measurements of sea water samples collected from different regions of the world ocean and was given in 1969 by UNESCO as SA (‰) = 1. 80655 Chlorinity (‰) SA is called as “Absolute Salinity”, unit: ppt chlorinity Cl is defined as "the mass of silver required to precipitate completely the halogens in 0. 328 523 4 kg of the sea-water sample. "

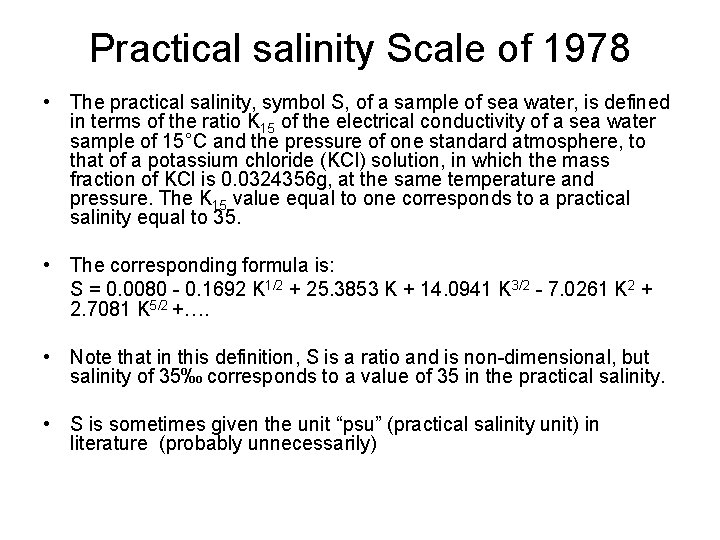
Practical salinity Scale of 1978 • The practical salinity, symbol S, of a sample of sea water, is defined in terms of the ratio K 15 of the electrical conductivity of a sea water sample of 15°C and the pressure of one standard atmosphere, to that of a potassium chloride (KCl) solution, in which the mass fraction of KCl is 0. 0324356 g, at the same temperature and pressure. The K 15 value equal to one corresponds to a practical salinity equal to 35. • The corresponding formula is: S = 0. 0080 - 0. 1692 K 1/2 + 25. 3853 K + 14. 0941 K 3/2 - 7. 0261 K 2 + 2. 7081 K 5/2 +…. • Note that in this definition, S is a ratio and is non-dimensional, but salinity of 35‰ corresponds to a value of 35 in the practical salinity. • S is sometimes given the unit “psu” (practical salinity unit) in literature (probably unnecessarily)

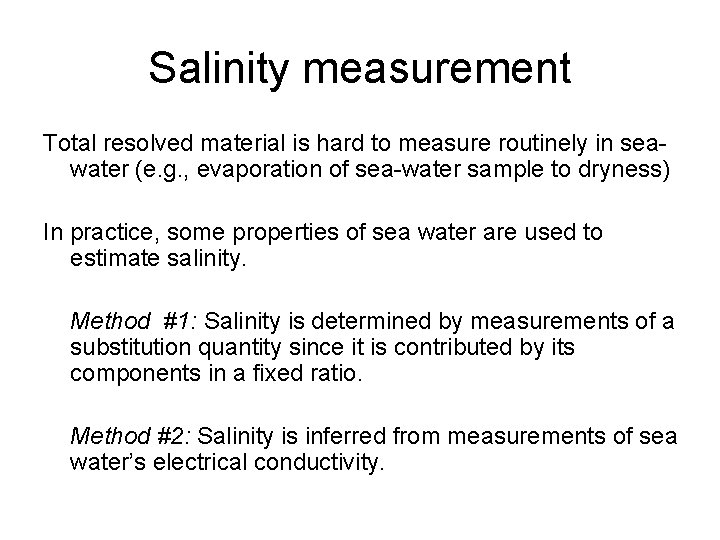
Salinity measurement Total resolved material is hard to measure routinely in seawater (e. g. , evaporation of sea-water sample to dryness) In practice, some properties of sea water are used to estimate salinity. Method #1: Salinity is determined by measurements of a substitution quantity since it is contributed by its components in a fixed ratio. Method #2: Salinity is inferred from measurements of sea water’s electrical conductivity.

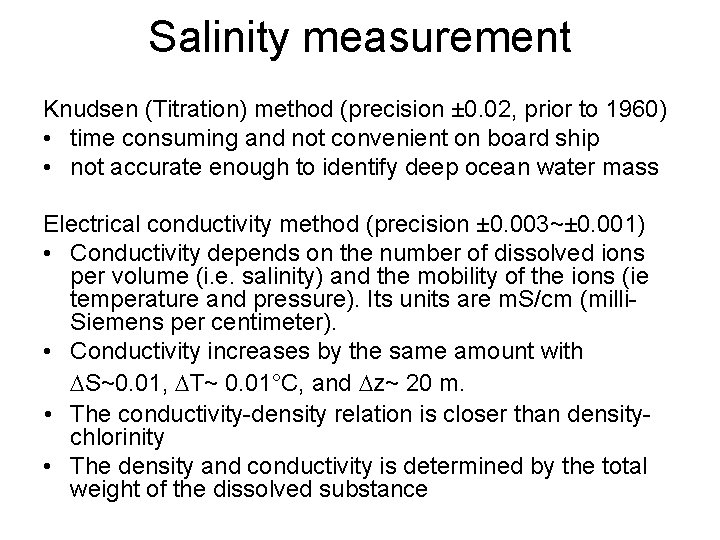
Salinity measurement Knudsen (Titration) method (precision ± 0. 02, prior to 1960) • time consuming and not convenient on board ship • not accurate enough to identify deep ocean water mass Electrical conductivity method (precision ± 0. 003~± 0. 001) • Conductivity depends on the number of dissolved ions per volume (i. e. salinity) and the mobility of the ions (ie temperature and pressure). Its units are m. S/cm (milli. Siemens per centimeter). • Conductivity increases by the same amount with S~0. 01, T~ 0. 01°C, and z~ 20 m. • The conductivity-density relation is closer than densitychlorinity • The density and conductivity is determined by the total weight of the dissolved substance

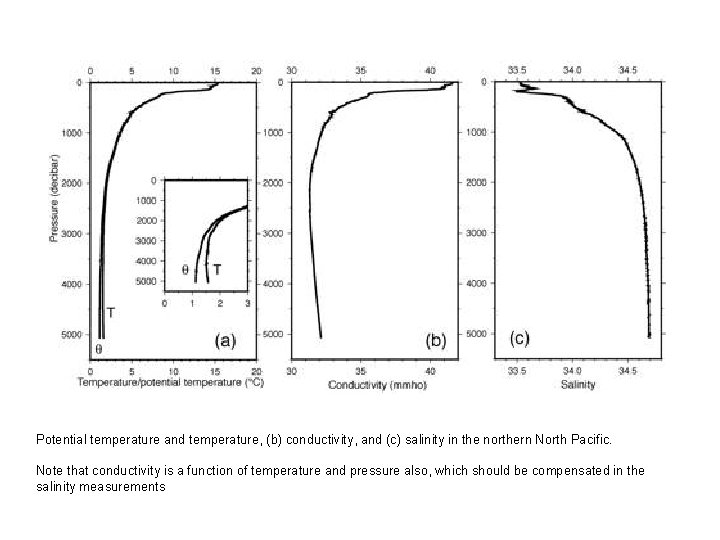
Potential temperature and temperature, (b) conductivity, and (c) salinity in the northern North Pacific. Note that conductivity is a function of temperature and pressure also, which should be compensated in the salinity measurements

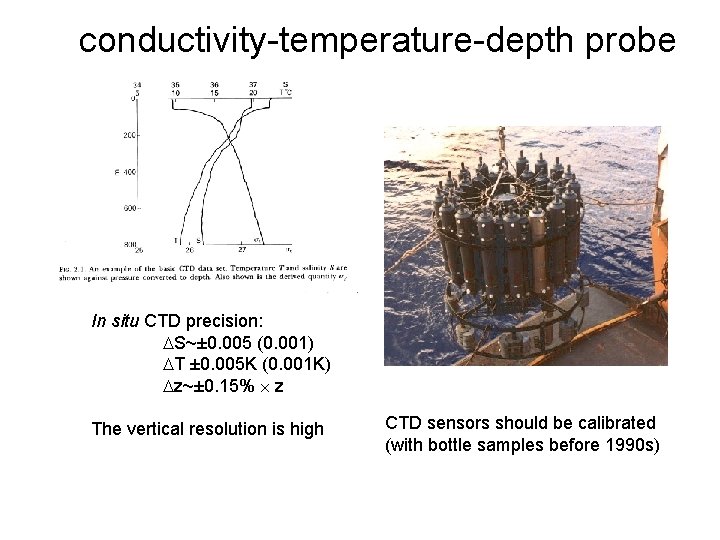
conductivity-temperature-depth probe In situ CTD precision: S~± 0. 005 (0. 001) T ± 0. 005 K (0. 001 K) z~± 0. 15% z The vertical resolution is high CTD sensors should be calibrated (with bottle samples before 1990 s)

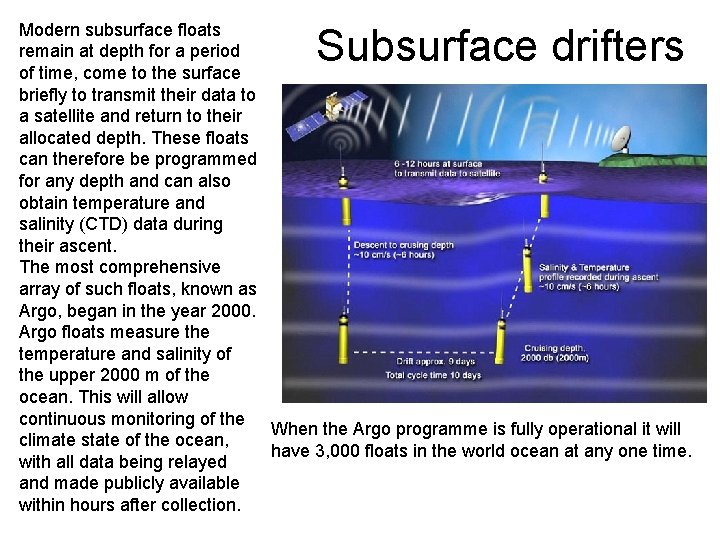
Modern subsurface floats remain at depth for a period of time, come to the surface briefly to transmit their data to a satellite and return to their allocated depth. These floats can therefore be programmed for any depth and can also obtain temperature and salinity (CTD) data during their ascent. The most comprehensive array of such floats, known as Argo, began in the year 2000. Argo floats measure the temperature and salinity of the upper 2000 m of the ocean. This will allow continuous monitoring of the When the Argo programme is fully operational it will climate state of the ocean, have 3, 000 floats in the world ocean at any one time. with all data being relayed and made publicly available within hours after collection. Subsurface drifters

Density ( , kg/m 3) • Determine the depth a water mass settles in equilibrium. • Determine the large scale circulation. • changes in the ocean is small. 1020 -1070 kg/m 3 (depth 0~10, 000 m) • increases with p (the greatest effect) ignoring p effect: ~1020. 0 -1030. 0 kg/m 3 1027. 7 -1027. 9 kg/m 3 for 50% of ocean • increases with S. decreases with T most of the time. • is usually not directly measured but determined from T, S, and p

Density anomaly Since the first two digits of never change, a new quantity is defined as s, t, p = – 1000 kg/m 3 called as “in-situ density anomaly”. (1000 kg/m 3 is for freshwater at 4 o. C) Atmospheric-pressure density anomaly (Sigma-tee) t = s, t, 0 – 1000 kg/m (note: s and t are in situ at the depth of measurement)

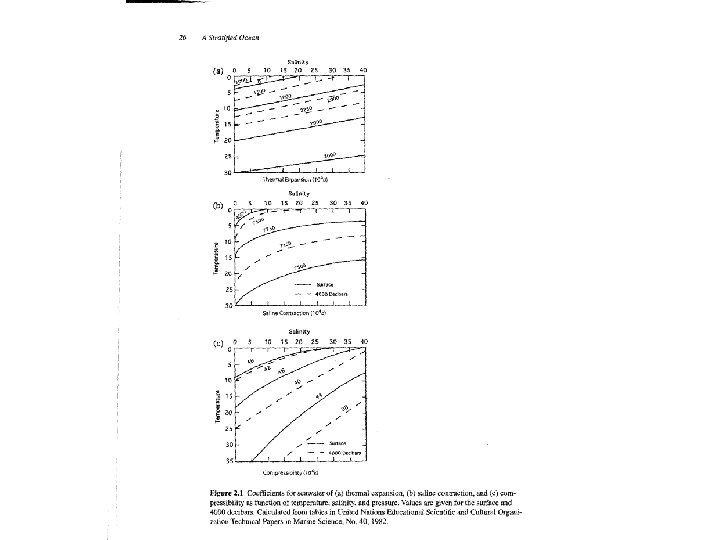
Equation of State The dependence of density (or ) on temperature T, salinity S and pressure p is the Equation of State of Sea Water. Bulk modulus K=1/ , is compressibility. , C speed of the sound in sea water. = (T, S, p) is determined by laboratory experiments. International Equation of State (1980) is the most widely used density formula now. • This equation uses T in °C, S from the Practical Salinity Scale and p in dbar (1 dbar = 104 Pascal = 104 N m-2) and gives in kg m 3. • Range: -2 o. C≤ T ≤ 40 o. C, 0 ≤ S ≤ 40, 0 ≤ p ≤ 105 k. Pa (depth, 0 to 10, 000 m) • Accuracy: 5 x 10 -6 (relative to pure water, ± 0. 005) • Polynomial expressions of (S, t, 0) (15 terms) and K(S, t, p) (27 terms) get accuracy of 9 x 10 -6.

Simple formula: (1) accuracy: ± 0. 5 kg/m 3 where 0=1027 kg/m 3, T 0=10 o. C, S 0=35 psu, a=-0. 15 kg/m 3 o. C, b=0. 78 kg/m 3, k=4. 5 x 10 -3 /dbar (2) where For 30≤S≤ 40, -2≤T≤ 30, p≤ 6 km, good to 0. 16 kg/m 3 For 0 ≤S≤ 40, good to 0. 3 kg/m 3


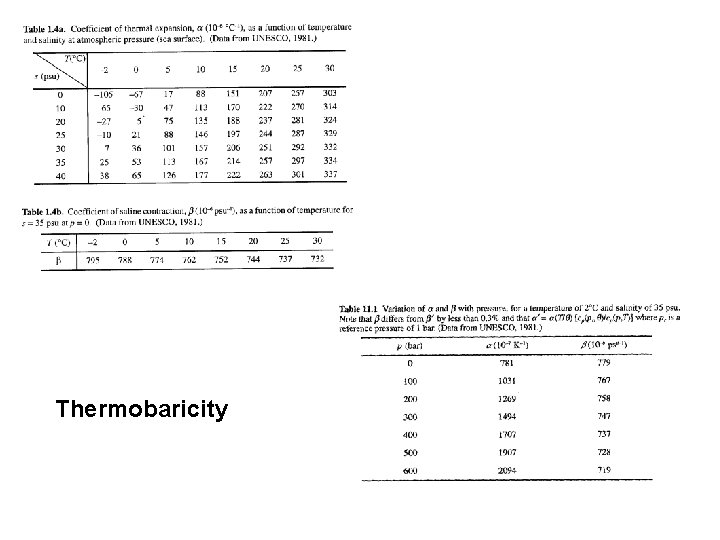
Thermobaricity


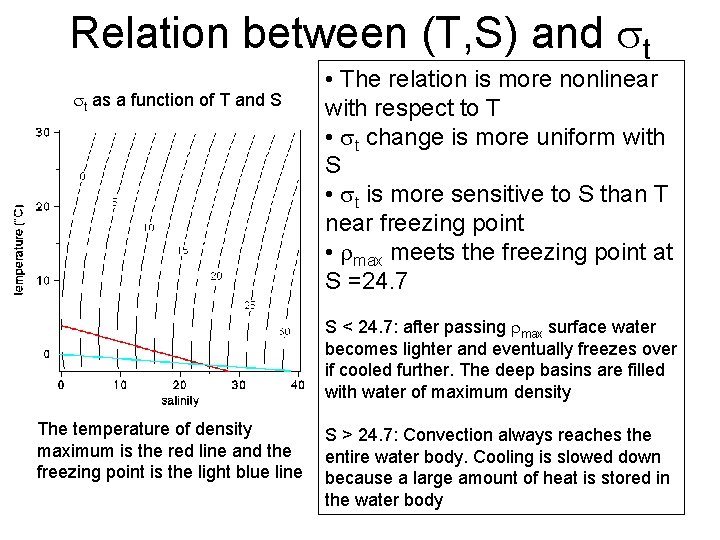
Relation between (T, S) and t t as a function of T and S • The relation is more nonlinear with respect to T • t change is more uniform with S • t is more sensitive to S than T near freezing point • max meets the freezing point at S =24. 7 S < 24. 7: after passing max surface water becomes lighter and eventually freezes over if cooled further. The deep basins are filled with water of maximum density The temperature of density maximum is the red line and the freezing point is the light blue line S > 24. 7: Convection always reaches the entire water body. Cooling is slowed down because a large amount of heat is stored in the water body

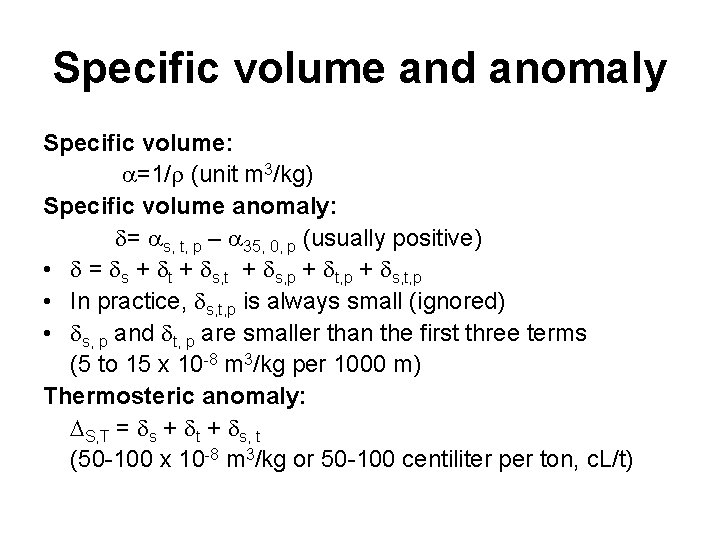
Specific volume and anomaly Specific volume: =1/ (unit m 3/kg) Specific volume anomaly: = s, t, p – 35, 0, p (usually positive) • = s + t + s, p + t, p + s, t, p • In practice, s, t, p is always small (ignored) • s, p and t, p are smaller than the first three terms (5 to 15 x 10 -8 m 3/kg per 1000 m) Thermosteric anomaly: S, T = s + t + s, t (50 -100 x 10 -8 m 3/kg or 50 -100 centiliter per ton, c. L/t)

Converting formula for S, T and t : Since (35, 0, 0)=0. 97266 x 10 -3 m 3/kg, , m 3/kg For 23 ≤ t ≤ 28, , accurate to 0. 1 accurate to 1 in c. L/ton For most part of the ocean, 25. 5 ≤ t≤ 28. 5. Correspondingly, 250 c. L/ton ≥ S, T ≥ -50 c. L/ton