CONCENTRATION The specific amount of solute grams dissolved








- Slides: 12

CONCENTRATION The specific amount of solute (grams) dissolved in a specific amount of solvent (millilitres) Ex. 18 g / 142 m. L 18 g of a solute was dissolved in 142 m. L of solvent

Can also be expressed as a % Ex. Vinegar – 5% acetic acid 5 m. L out of 100 m. L is real acid The rest (95 ml) is water Very low concentrations may be stated in ‘parts per million’ or ppm Often used for pollutants Ex. DDT is found in the environment @ 2 ppm 2 parts of DDT is found in 1 million parts of the environment

2 main types of concentrations: 1) Concentrated Large amount of solute in the solvent Ex. Frozen orange juice concentrate Large amount of orange solids in water

2) Diluted Small amount of solute in the solvent Ex. Orange juice made from concentrate Small amount of orange solids in a large amount of water

How could you make the diluted solution more concentrated? How could you make the concentrated solution more dilute?

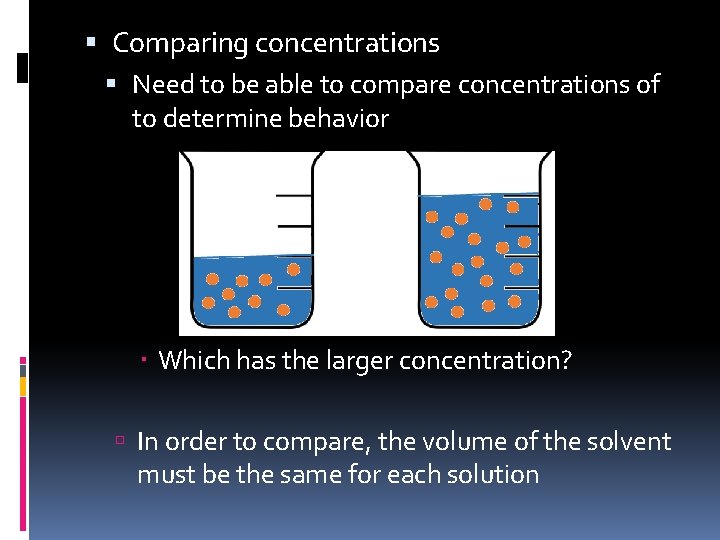
Comparing concentrations Need to be able to compare concentrations of to determine behavior Which has the larger concentration? In order to compare, the volume of the solvent must be the same for each solution

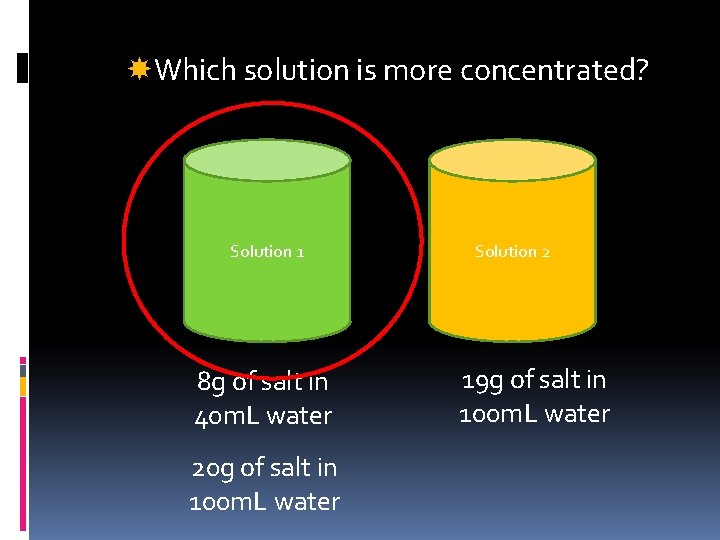
Which solution is more concentrated? Solution 1 8 g of salt in 40 m. L water 20 g of salt in 100 m. L water Solution 2 19 g of salt in 100 m. L water

Which solution is more concentrated? Solution 1 14 g of salt in 65 m. L water Solution 2 20 g of salt in 85 m. L water

Who’s Is Stronger? Each group will make 4 different concentration of Tang Label each cup (A 1, A 2, etc) Measure out solute and solvent; mix Groups will then rotate to another group’s drinks and order the drinks from most to least concentrated based on appearance Then check with calculations to determine if they were correct Enjoy!

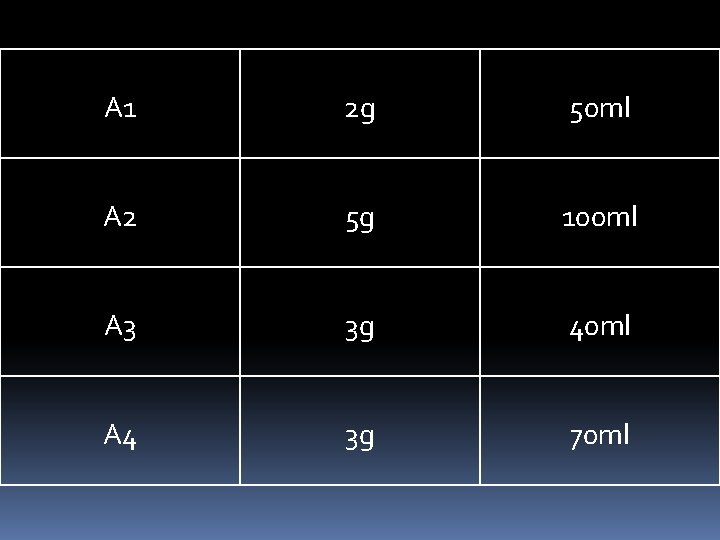
A 1 2 g 50 ml A 2 5 g 100 ml A 3 3 g 40 ml A 4 3 g 70 ml

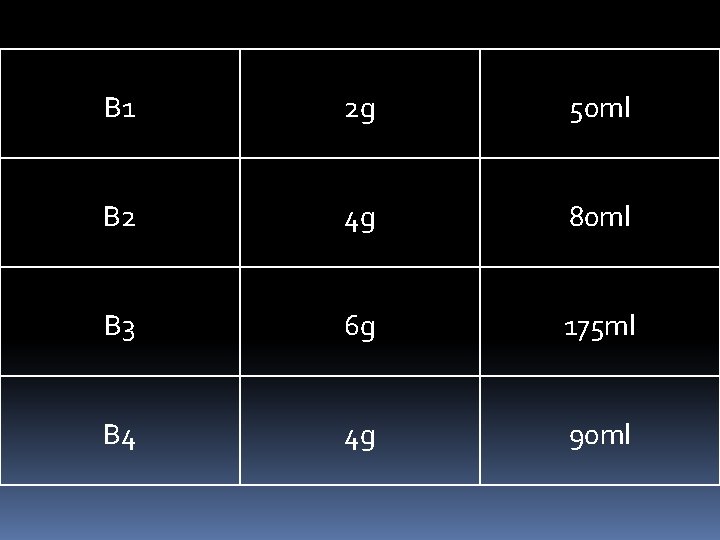
B 1 2 g 50 ml B 2 4 g 80 ml B 3 6 g 175 ml B 4 4 g 90 ml

A Taste of Concentration Task - Create a glass of Tang that has your ideal concentration Procedure Start by making a solution of 5 g/200 ml Increase the concentration until you have reached your ideal drink Add 1 g increments of iced tea powder Maximum concentration = 15 g/200 ml Calculate your ideal concentration in g/200 ml, g/ml, and % Enjoy!