Solution Concentration the quantity of solute dissolved in


- Slides: 10

Solution Concentration the quantity of solute dissolved in a specific quantity of solvent or solution S-C-9 -3_Concentrations Presentation

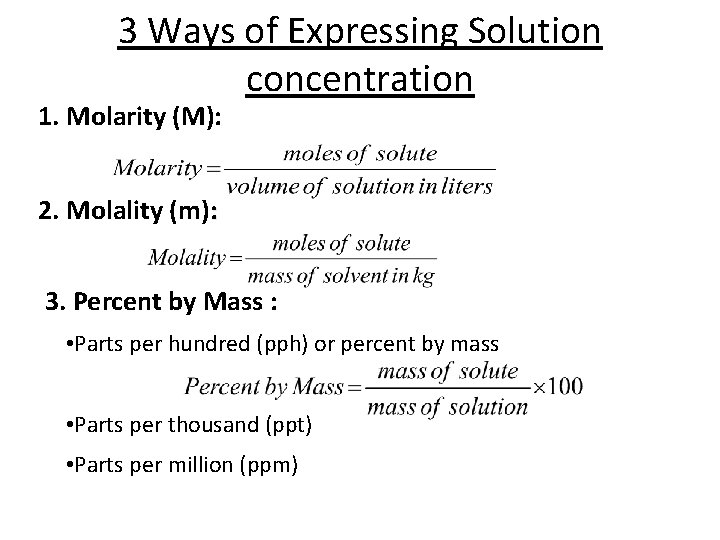
3 Ways of Expressing Solution concentration 1. Molarity (M): 2. Molality (m): 3. Percent by Mass : • Parts per hundred (pph) or percent by mass • Parts per thousand (ppt) • Parts per million (ppm)

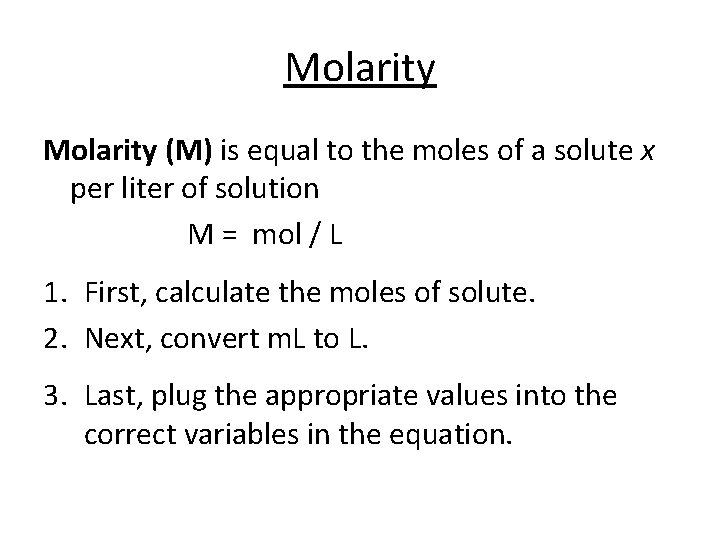
Molarity (M) is equal to the moles of a solute x per liter of solution M = mol / L 1. First, calculate the moles of solute. 2. Next, convert m. L to L. 3. Last, plug the appropriate values into the correct variables in the equation.

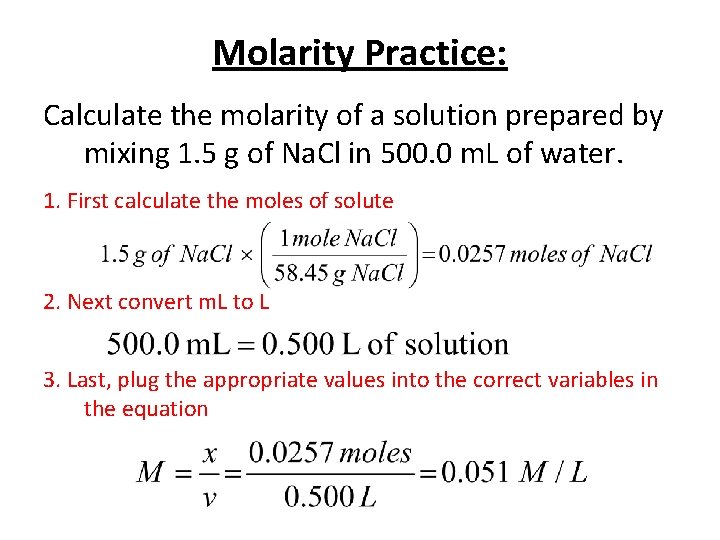
Molarity Practice: Calculate the molarity of a solution prepared by mixing 1. 5 g of Na. Cl in 500. 0 m. L of water. 1. First calculate the moles of solute 2. Next convert m. L to L 3. Last, plug the appropriate values into the correct variables in the equation

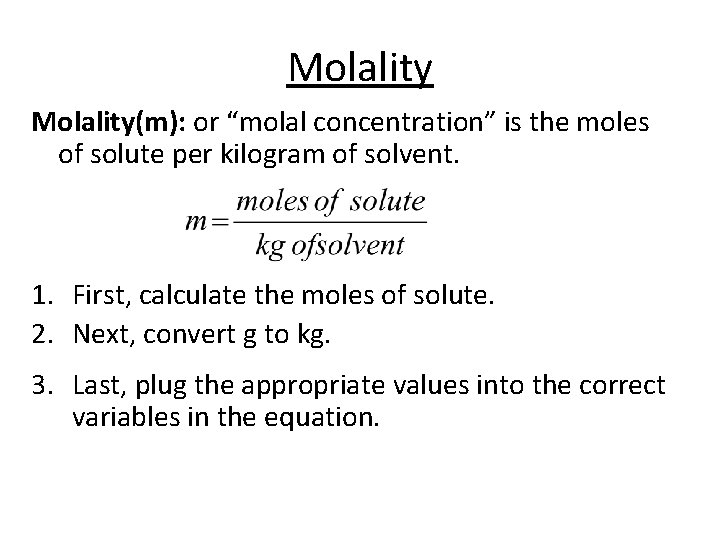
Molality(m): or “molal concentration” is the moles of solute per kilogram of solvent. 1. First, calculate the moles of solute. 2. Next, convert g to kg. 3. Last, plug the appropriate values into the correct variables in the equation.

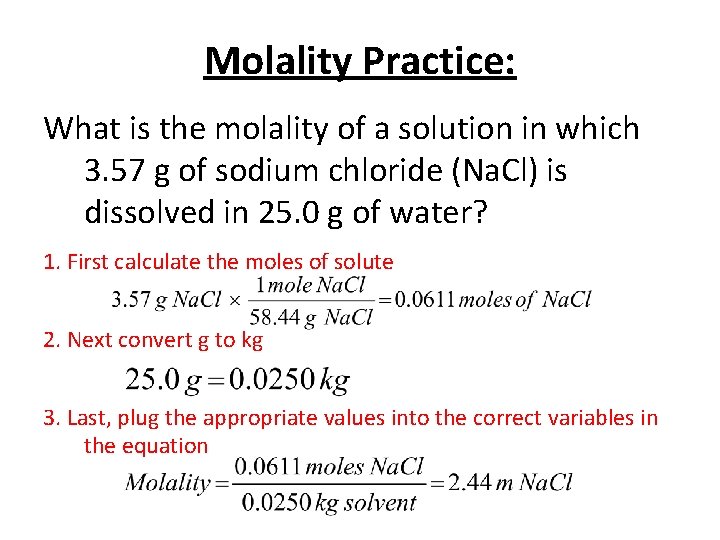
Molality Practice: What is the molality of a solution in which 3. 57 g of sodium chloride (Na. Cl) is dissolved in 25. 0 g of water? 1. First calculate the moles of solute 2. Next convert g to kg 3. Last, plug the appropriate values into the correct variables in the equation

Percent by Mass: Describes the amount of solute dissolved in 100, parts of solution (g solute per H 20) Need: – The mass of the solute in the solution. – The mass of the solution.

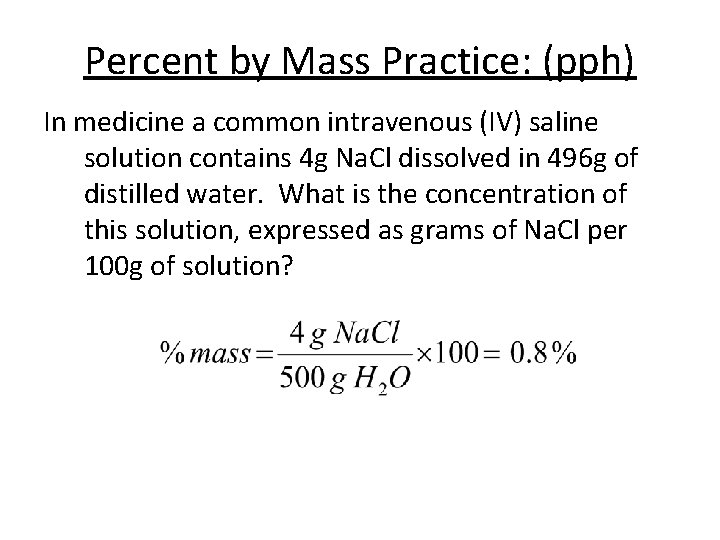
Percent by Mass Practice: (pph) In medicine a common intravenous (IV) saline solution contains 4 g Na. Cl dissolved in 496 g of distilled water. What is the concentration of this solution, expressed as grams of Na. Cl per 100 g of solution?

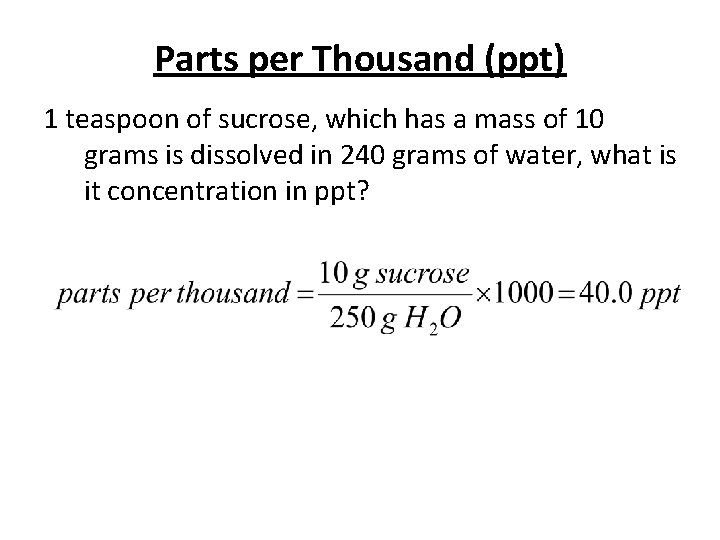
Parts per Thousand (ppt) 1 teaspoon of sucrose, which has a mass of 10 grams is dissolved in 240 grams of water, what is it concentration in ppt?

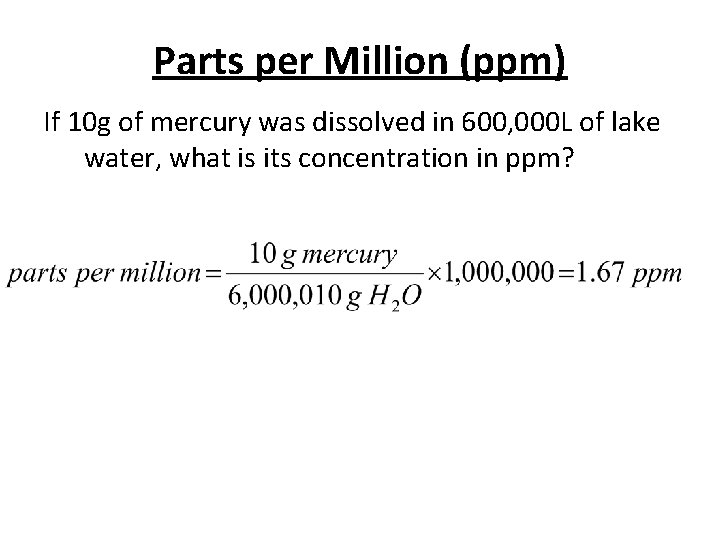
Parts per Million (ppm) If 10 g of mercury was dissolved in 600, 000 L of lake water, what is its concentration in ppm?