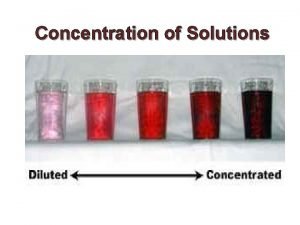
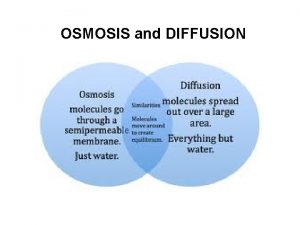
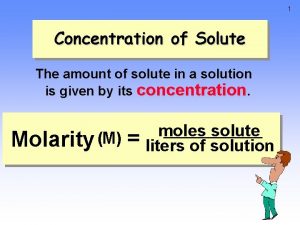
Concentration amount of solute per quantity of solvent





- Slides: 12


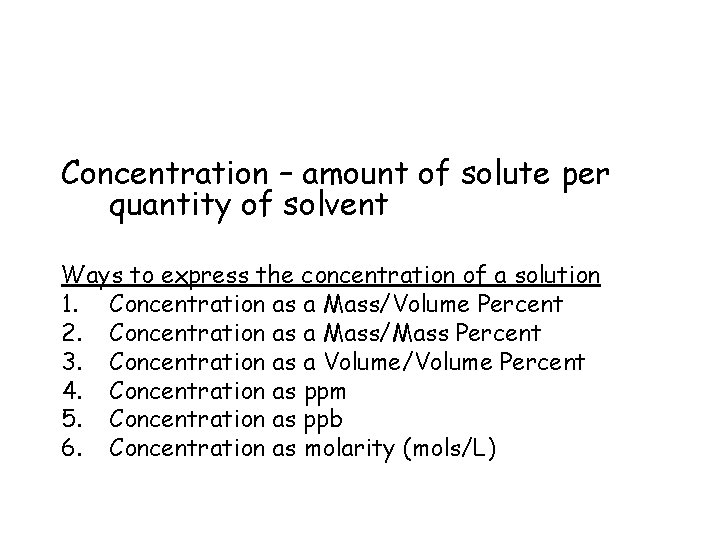
Concentration – amount of solute per quantity of solvent Ways to express the concentration of a solution 1. Concentration as a Mass/Volume Percent 2. Concentration as a Mass/Mass Percent 3. Concentration as a Volume/Volume Percent 4. Concentration as ppm 5. Concentration as ppb 6. Concentration as molarity (mols/L)

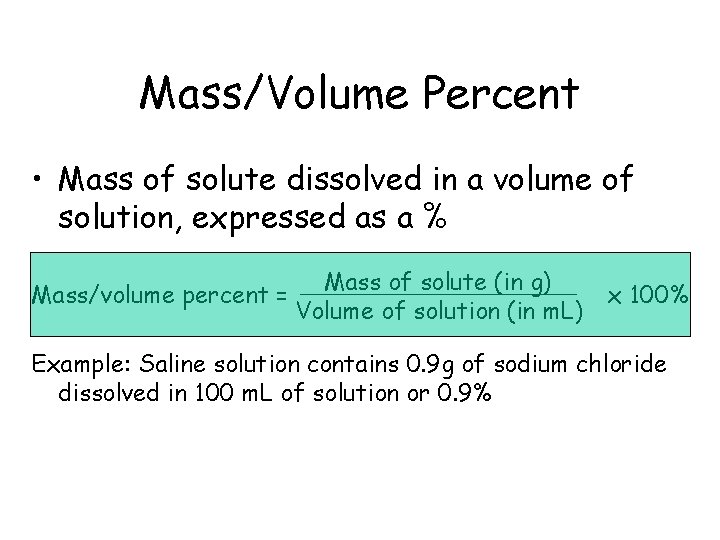
Mass/Volume Percent • Mass of solute dissolved in a volume of solution, expressed as a % Mass/volume percent = Mass of solute (in g) Volume of solution (in m. L) x 100% Example: Saline solution contains 0. 9 g of sodium chloride dissolved in 100 m. L of solution or 0. 9%

A pharmacist adds 2. 00 m. L of distilled water to 4. 00 g of a powdered drug. The final volume of the solution is 3. 00 m. L. a. What is the percent (m/v) of the solution? mass of solute (in g) Mass/volume percent= x 100% Volume of solution (in m. L) = 4. 00 g x 100% 3. 00 m. L = 133%

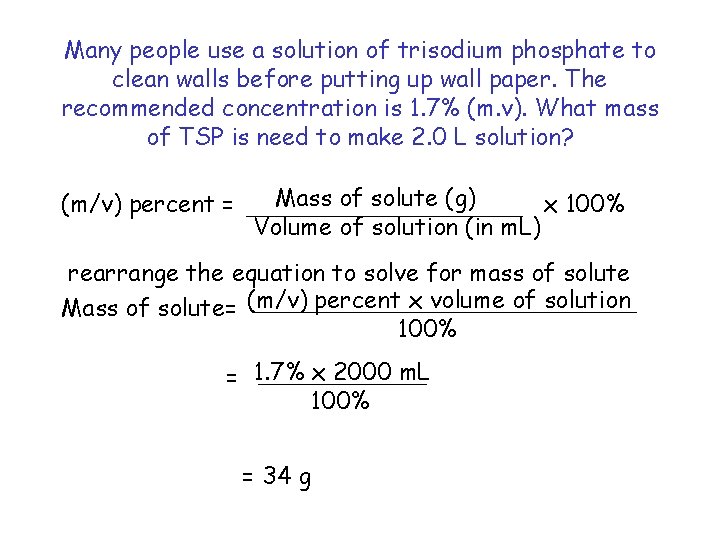
Many people use a solution of trisodium phosphate to clean walls before putting up wall paper. The recommended concentration is 1. 7% (m. v). What mass of TSP is need to make 2. 0 L solution? (m/v) percent = Mass of solute (g) x 100% Volume of solution (in m. L) rearrange the equation to solve for mass of solute Mass of solute= (m/v) percent x volume of solution 100% = 1. 7% x 2000 m. L 100% = 34 g

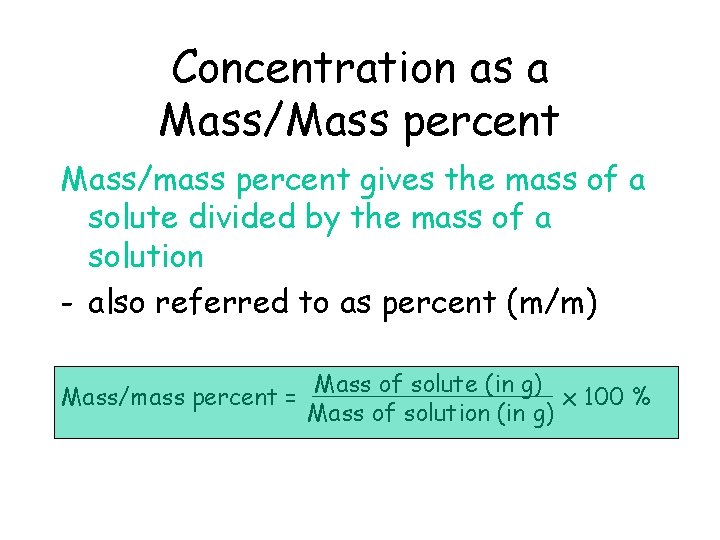
Concentration as a Mass/Mass percent Mass/mass percent gives the mass of a solute divided by the mass of a solution - also referred to as percent (m/m) Mass/mass percent = Mass of solute (in g) x 100 % Mass of solution (in g)

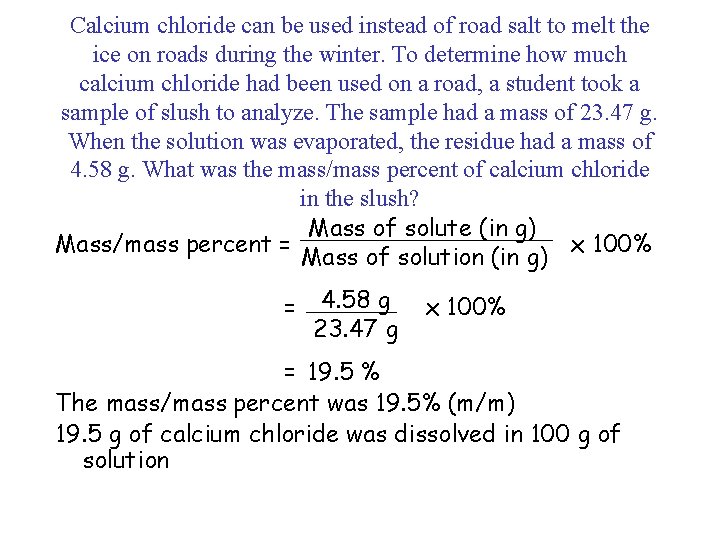
Calcium chloride can be used instead of road salt to melt the ice on roads during the winter. To determine how much calcium chloride had been used on a road, a student took a sample of slush to analyze. The sample had a mass of 23. 47 g. When the solution was evaporated, the residue had a mass of 4. 58 g. What was the mass/mass percent of calcium chloride in the slush? Mass of solute (in g) Mass/mass percent = x 100% Mass of solution (in g) = 4. 58 g 23. 47 g x 100% = 19. 5 % The mass/mass percent was 19. 5% (m/m) 19. 5 g of calcium chloride was dissolved in 100 g of solution

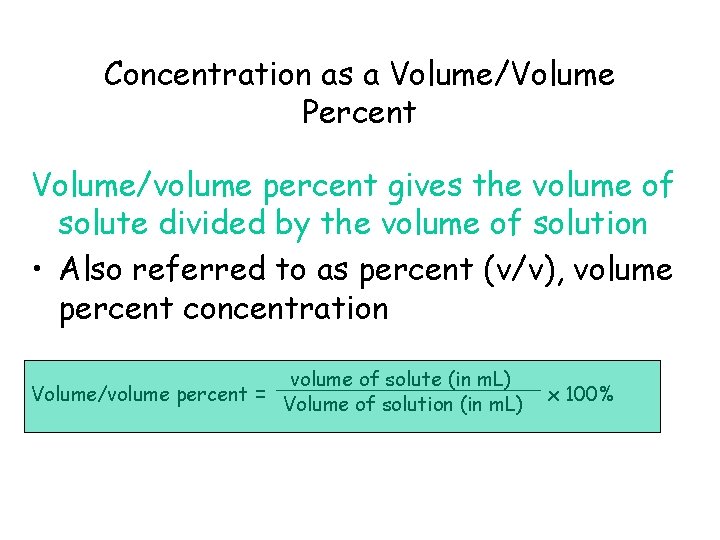
Concentration as a Volume/Volume Percent Volume/volume percent gives the volume of solute divided by the volume of solution • Also referred to as percent (v/v), volume percent concentration Volume/volume percent = volume of solute (in m. L) Volume of solution (in m. L) x 100%

Rubbing alcohol is commonly used as an antiseptic for small cuts. It is sold as 70% (v/v) solution of isopropyl alcohol in water. What volume of isopropyl alcohol is used to make 500 m. L of rubbing alcohol? Using this equation, Volume/volume percent = volume of solute x 100 % Volume of solution Rearrange to solve for volume of solute: Volume/volume % x volume of solution 100% 70% x 500 m. L = 100% Volume of solute = = 350 m. L of isopropyl alcohol is used to make 500 m. L of 70% (v/v) rubbing alcohol.

Concentration in parts per million or parts per billion ppm – parts per million (106)(one part solute per million parts of solution) ppb – parts per billion (109)(one part solute per billion parts of solution) Usually mass/mass relationships ie. The mass of solute compared with the mass of solution. mass of solute (g) x 10 6 ppm = Mass of solution (g) OR Mass of solute Mass of solution = xg 106 g of solution

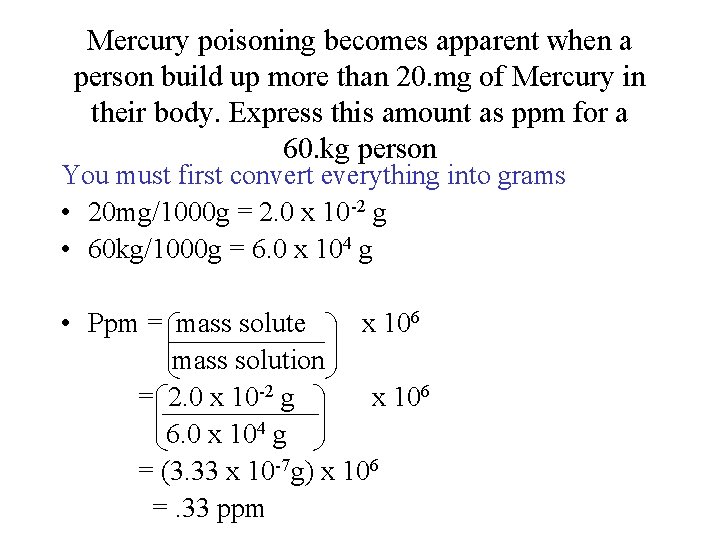
Mercury poisoning becomes apparent when a person build up more than 20. mg of Mercury in their body. Express this amount as ppm for a 60. kg person You must first convert everything into grams • 20 mg/1000 g = 2. 0 x 10 -2 g • 60 kg/1000 g = 6. 0 x 104 g • Ppm = mass solute x 106 mass solution = 2. 0 x 10 -2 g x 106 6. 0 x 104 g = (3. 33 x 10 -7 g) x 106 =. 33 ppm

Homework • 8. 6 Homework- molarity • 8. 7 Homework % concentration
 A measure of the amount of solute dissolved in a solvent
A measure of the amount of solute dissolved in a solvent Solutes and solvents grade 7 science
Solutes and solvents grade 7 science Solute vs solvent
Solute vs solvent Sweet tea solute and solvent
Sweet tea solute and solvent Saturated vs unsaturated solution
Saturated vs unsaturated solution How is the solute being dissolved in the given solvent
How is the solute being dissolved in the given solvent Solute vs solvent
Solute vs solvent Is a solution a homogeneous mixture
Is a solution a homogeneous mixture Chapter 1 matter and change worksheet answers
Chapter 1 matter and change worksheet answers Lemonade solute and solvent
Lemonade solute and solvent Homogeneous mixture of a solute in a solvent
Homogeneous mixture of a solute in a solvent He took his seat quietly
He took his seat quietly Solute vs solvent
Solute vs solvent