Salinity Salinity is the total amount of dissolved








- Slides: 10

Salinity • Salinity is the total amount of dissolved salts in water; grams of salts per kilogram of water (g/kg) or as parts per thousand (ppt). • Seawater has 11 major constituents that make up more than 99. 99% of all dissolved materials. • Although salinity may vary, the major constituents are well mixed and present in the same relative proportions.

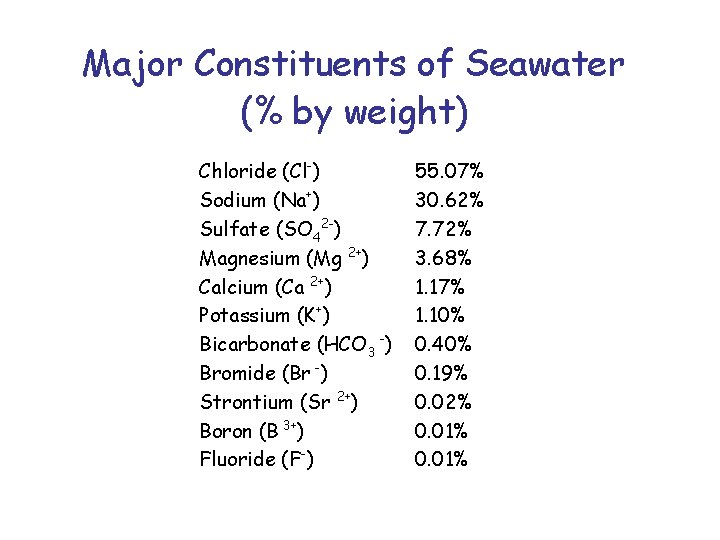
Major Constituents of Seawater (% by weight) Chloride (Cl-) Sodium (Na+) Sulfate (SO 42 -) Magnesium (Mg 2+) Calcium (Ca 2+) Potassium (K+) Bicarbonate (HCO 3 -) Bromide (Br -) Strontium (Sr 2+) Boron (B 3+) Fluoride (F-) 55. 07% 30. 62% 7. 72% 3. 68% 1. 17% 1. 10% 0. 40% 0. 19% 0. 02% 0. 01%

Why Study Salinity… • Determines the distribution of plants and animals that live in the ocean. • Affects other properties of seawater, such as its density and the amount of dissolved oxygen.

Significant Values • The average salinity of the world’s oceans is 35 ppt. • Freshwater has a salinity of <1 ppt. • Inshore waters with salinity values between 1 25 ppt are called brackish. • Waters with salinity greater than 40 ppt are called hypersaline.

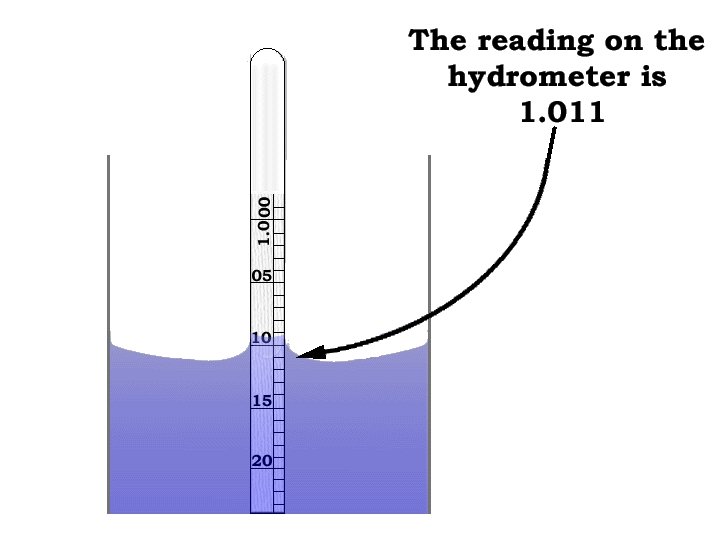
Salinity Hydrometer Method • Addition of salts to pure water causes an increase in density. Salinity can be calculated by measuring the specific gravity of a water sample using a hydrometer, correcting for the effect of temperature and converting the readings to salinity by using conversion tables. Specific Gravity = density of sample Density of pure water

Hydrometer Reading • • Be sure hydrometer is clean Fill 500 m. L graduated cylinder with sample water Determine the temperature of your sample Place the hydrometer in cylinder and let settle. It should not touch the cylinder walls, and should be read from the bottom of the meniscus. • Read the specific gravity from the hydrometer scale • Using the specific gravity and temperature values, determine salinity from salinity table in Teacher’s Guide • Read three times. The values should be within 2 ppt of the average. Discard outliers.


Calibration - Hydrometer Method • 35 ppt standard: – Measure out 17. 5 g Na. Cl (table salt) and pour into a 500 -m. L graduated cylinder. – Fill the cylinder to the line with distilled water and carefully swirl the solution to mix the standard, until all salt crystals have dissolved. – Pour the solution into a 1 -quart plastic bottle and label. • Prepare a blank using 500 ml of distilled water. • Follow the directions for a water sample. • Check technique every six months.

Salinity titration method • The amount of halogens (chloride, bromide, iodine, and fluoride) in the water sample (chlorinity) is determined using a silver nitrate titration method. The salinity of the sample can then be calculated using the following formula: Salinity (ppt) = 1. 80655 x Chlorinity (ppt) • Use a test kit that meet specifications described in the Tool Kit in the GLOBE Teacher’s Guide • Follow the instructions in the test kit to take three measurements. Values should be within the precision stated in the test kit specifications. Discard outliers and retake measurements.

Calibration - Titration Method • Concentration of standard reflect composition of seawater. • 38. 6 ppt sea water titration standard: – Measure out 17. 5 g Na. Cl (table salt) and pour this into a 500 -m. L graduated cylinder. – Fill the cylinder to the line with distilled water and carefully swirl the solution to mix the standard, until all salt crystals have dissolved. – Pour the solution into a 1 -quart plastic bottle and label. • Follow directions for a water sample. • Calibrate every six months to check technique.