Safety of Quadrivalent Human Papillomavirus HPV 4 Vaccine









- Slides: 28

Safety of Quadrivalent Human Papillomavirus (HPV 4) Vaccine: What Providers Should Know National Immunization Conference March 28, 2012 Julianne Gee, MPH Immunization Safety Office Centers for Disease Control and Prevention

Presentation Outline • Describe sources of HPV 4 safety data • Review of US surveillance HPV 4 safety data • Review findings from a recent Vaccine Safety Datalink (VSD) study • Review findings from 2011 Institute of Medicine’s report of adverse events and vaccines

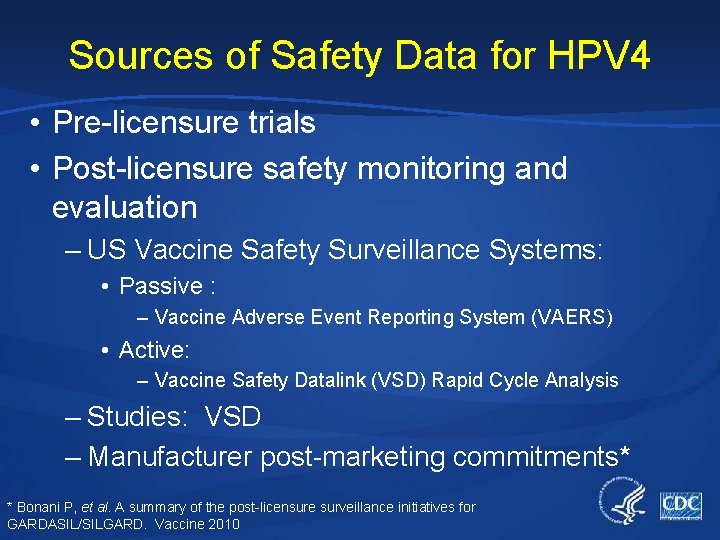
Sources of Safety Data for HPV 4 • Pre-licensure trials • Post-licensure safety monitoring and evaluation – US Vaccine Safety Surveillance Systems: • Passive : – Vaccine Adverse Event Reporting System (VAERS) • Active: – Vaccine Safety Datalink (VSD) Rapid Cycle Analysis – Studies: VSD – Manufacturer post-marketing commitments* * Bonani P, et al. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine 2010

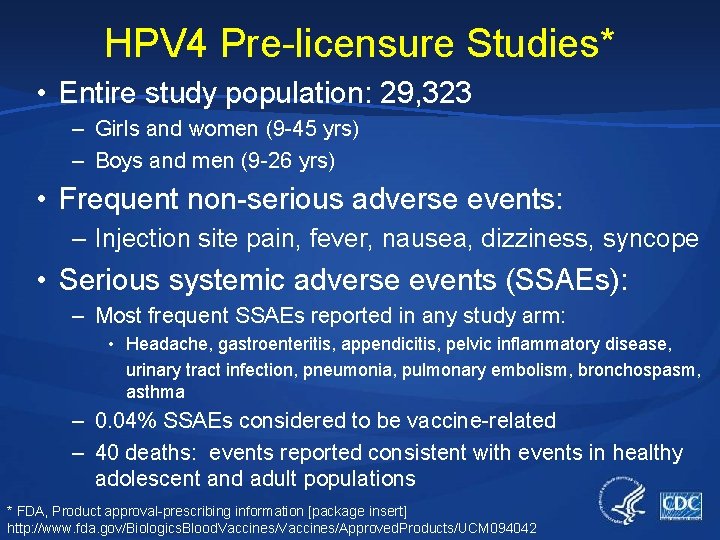
HPV 4 Pre-licensure Studies* • Entire study population: 29, 323 – Girls and women (9 -45 yrs) – Boys and men (9 -26 yrs) • Frequent non-serious adverse events: – Injection site pain, fever, nausea, dizziness, syncope • Serious systemic adverse events (SSAEs): – Most frequent SSAEs reported in any study arm: • Headache, gastroenteritis, appendicitis, pelvic inflammatory disease, urinary tract infection, pneumonia, pulmonary embolism, bronchospasm, asthma – 0. 04% SSAEs considered to be vaccine-related – 40 deaths: events reported consistent with events in healthy adolescent and adult populations * FDA, Product approval-prescribing information [package insert] http: //www. fda. gov/Biologics. Blood. Vaccines/Approved. Products/UCM 094042

Vaccine Adverse Event Reporting System (VAERS) • National post-licensure passive surveillance system • Co-managed by CDC and FDA • Early warning system for vaccine safety surveillance – Monitors for known adverse events (AEs) – Detects signals for previously unrecognized or rare AEs – Generates hypotheses for further study • Limitations – – Reporting biases Incomplete data Lack of availability of denominator data Not designed to assess for causality

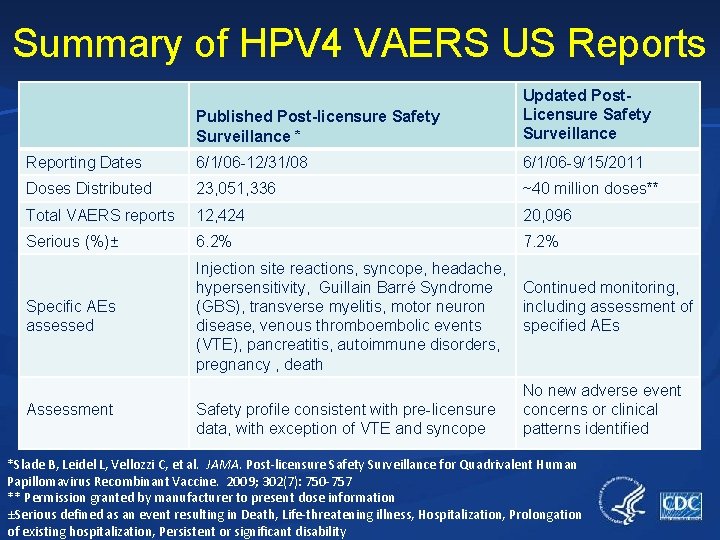
Summary of HPV 4 VAERS US Reports Published Post-licensure Safety Surveillance * Updated Post. Licensure Safety Surveillance Reporting Dates 6/1/06 -12/31/08 6/1/06 -9/15/2011 Doses Distributed 23, 051, 336 ~40 million doses** Total VAERS reports 12, 424 20, 096 Serious (%)± 6. 2% 7. 2% Specific AEs assessed Injection site reactions, syncope, headache, hypersensitivity, Guillain Barré Syndrome (GBS), transverse myelitis, motor neuron disease, venous thromboembolic events (VTE), pancreatitis, autoimmune disorders, pregnancy , death Assessment Safety profile consistent with pre-licensure data, with exception of VTE and syncope Continued monitoring, including assessment of specified AEs No new adverse event concerns or clinical patterns identified *Slade B, Leidel L, Vellozzi C, et al. JAMA. Post-licensure Safety Surveillance for Quadrivalent Human Papillomavirus Recombinant Vaccine. 2009; 302(7): 750 -757 ** Permission granted by manufacturer to present dose information ±Serious defined as an event resulting in Death, Life-threatening illness, Hospitalization, Prolongation of existing hospitalization, Persistent or significant disability

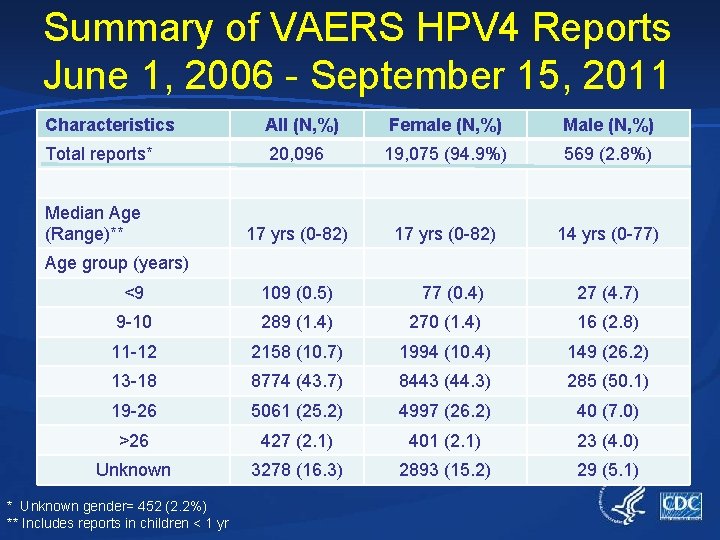
Summary of VAERS HPV 4 Reports June 1, 2006 - September 15, 2011 Characteristics All (N, %) Female (N, %) Male (N, %) Total reports* 20, 096 19, 075 (94. 9%) 569 (2. 8%) 17 yrs (0 -82) 14 yrs (0 -77) Median Age (Range)** Age group (years) <9 109 (0. 5) 77 (0. 4) 27 (4. 7) 9 -10 289 (1. 4) 270 (1. 4) 16 (2. 8) 11 -12 2158 (10. 7) 1994 (10. 4) 149 (26. 2) 13 -18 8774 (43. 7) 8443 (44. 3) 285 (50. 1) 19 -26 5061 (25. 2) 4997 (26. 2) 40 (7. 0) >26 427 (2. 1) 401 (2. 1) 23 (4. 0) Unknown 3278 (16. 3) 2893 (15. 2) 29 (5. 1) * Unknown gender= 452 (2. 2%) ** Includes reports in children < 1 yr

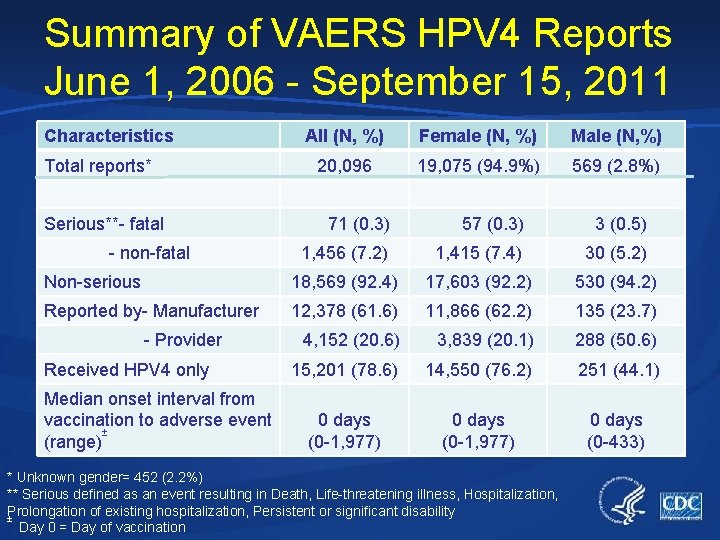
Summary of VAERS HPV 4 Reports June 1, 2006 - September 15, 2011 Characteristics Total reports* Serious**- fatal All (N, %) Female (N, %) Male (N, %) 20, 096 19, 075 (94. 9%) 569 (2. 8%) 71 (0. 3) 57 (0. 3) 3 (0. 5) 1, 456 (7. 2) 1, 415 (7. 4) 30 (5. 2) Non-serious 18, 569 (92. 4) 17, 603 (92. 2) 530 (94. 2) Reported by- Manufacturer 12, 378 (61. 6) 11, 866 (62. 2) 135 (23. 7) 4, 152 (20. 6) 3, 839 (20. 1) 288 (50. 6) 15, 201 (78. 6) 14, 550 (76. 2) 251 (44. 1) 0 days (0 -1, 977) 0 days (0 -433) - non-fatal - Provider Received HPV 4 only Median onset interval from vaccination to adverse event ± (range) * Unknown gender= 452 (2. 2%) ** Serious defined as an event resulting in Death, Life-threatening illness, Hospitalization, Prolongation of existing hospitalization, Persistent or significant disability ± Day 0 = Day of vaccination

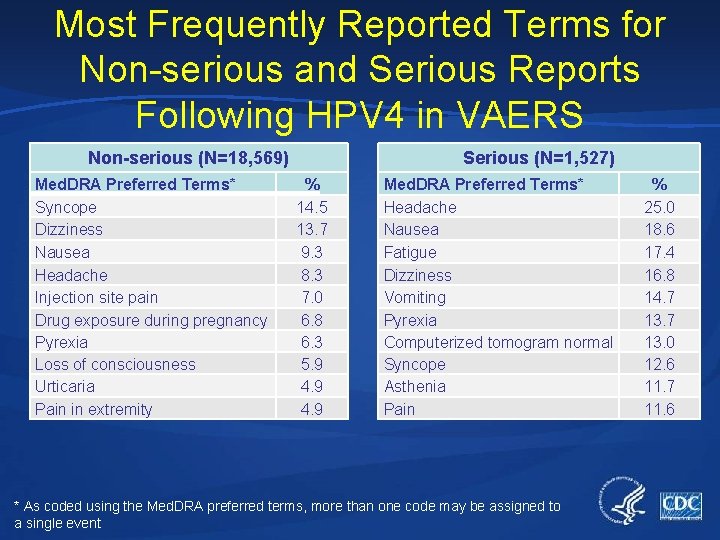
Most Frequently Reported Terms for Non-serious and Serious Reports Following HPV 4 in VAERS Non-serious (N=18, 569) Med. DRA Preferred Terms* Syncope Dizziness Nausea Headache Injection site pain Drug exposure during pregnancy Pyrexia Loss of consciousness Urticaria Pain in extremity Serious (N=1, 527) % 14. 5 13. 7 9. 3 8. 3 7. 0 6. 8 6. 3 5. 9 4. 9 Med. DRA Preferred Terms* Headache Nausea Fatigue Dizziness Vomiting Pyrexia Computerized tomogram normal Syncope Asthenia Pain * As coded using the Med. DRA preferred terms, more than one code may be assigned to a single event % 25. 0 18. 6 17. 4 16. 8 14. 7 13. 0 12. 6 11. 7 11. 6

: VAERS Serious Reports of Syncope Following HPV 4* • Total number of serious reports: 202 • Injuries resulting from syncopal event: – Fractures (nose, skull, maxillary) – Dental injuries – Contusions – Concussions – Intracranial hemorrhages (subdural hematoma, subarachnoid hemorrhage) • No reports of death resulting from injury following a vasovagal syncopal event * Unverified reports coded as syncope or syncope vasovagal

VAERS Reports of Anaphylaxis • Total number of reports: 43* – All female – Serious reports: 20 • Median age (range): 17 years (11 -27) • Median onset from vaccination to event: 0 days (0 -10) • Confirmed anaphylaxis: 12 – All treated and recovered • No confirmed deaths due to anaphylaxis *Reports coded with Med. DRA terms: anaphylactic shock, anaphylactic reaction, anaphylactoid shock, anaphylactoid reaction

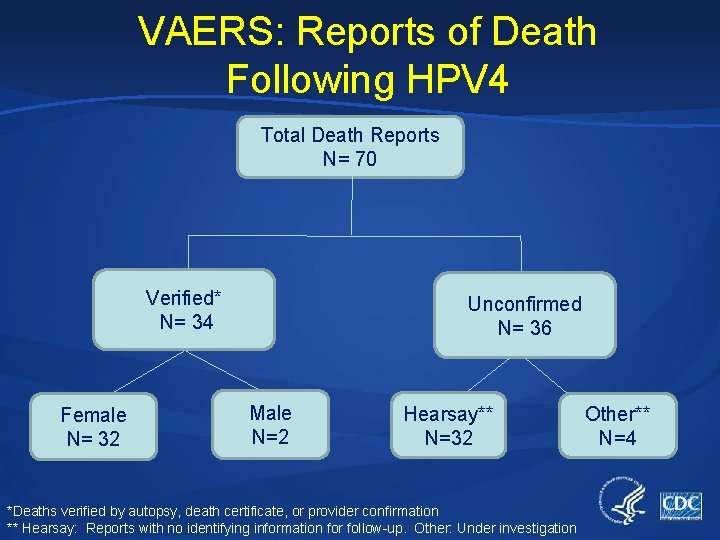
VAERS: Reports of Death Following HPV 4 Total Death Reports N= 70 Verified* N= 34 Female N= 32 Unconfirmed N= 36 Male N=2 Hearsay** N=32 *Deaths verified by autopsy, death certificate, or provider confirmation ** Hearsay: Reports with no identifying information for follow-up. Other: Under investigation Other** N=4

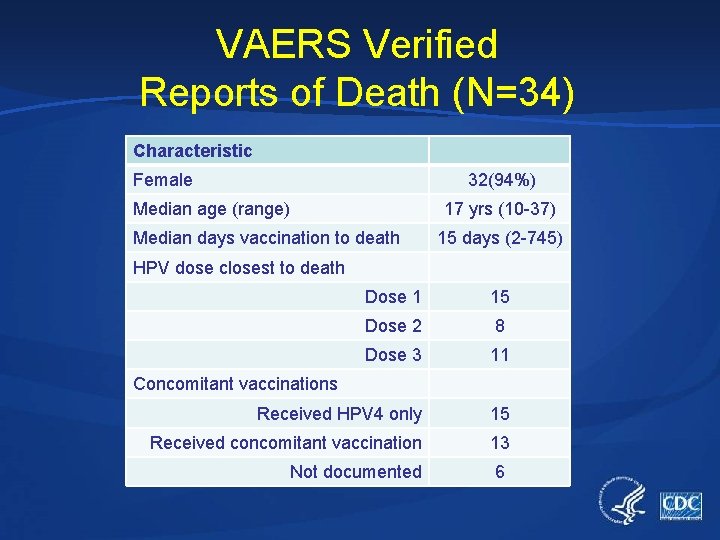
VAERS Verified Reports of Death (N=34) Characteristic Female 32(94%) Median age (range) 17 yrs (10 -37) Median days vaccination to death 15 days (2 -745) HPV dose closest to death Dose 1 15 Dose 2 8 Dose 3 11 Received HPV 4 only 15 Received concomitant vaccination 13 Not documented 6 Concomitant vaccinations

VAERS Verified Reports of Death • Reported causes of death after clinical review with median onset interval: • Neurological: 7 (seizures [5]; ALS [2]) – 53 days (13 -745) • Cardiac: 7 (arrhythmia [3]; myocarditis [3]; congenital) – 9 days (2 -25) • Pulmonary embolism: 4 – 14. 5 days (13 -181) • Infectious: 5 (Group A Strep [2]; N. meningitidis, MRSA; HIV-CNS vasculitis) – 29 (4 -117) • Other non-infectious: 4 (suicide; type 1 DM DKA; drug overdose; polymyositis) – 35 (2 -594) • Undetermined cause of death: 7 – 17 (2 -121) • No patterns in verified death events * Median onset interval from vaccination to death (range)

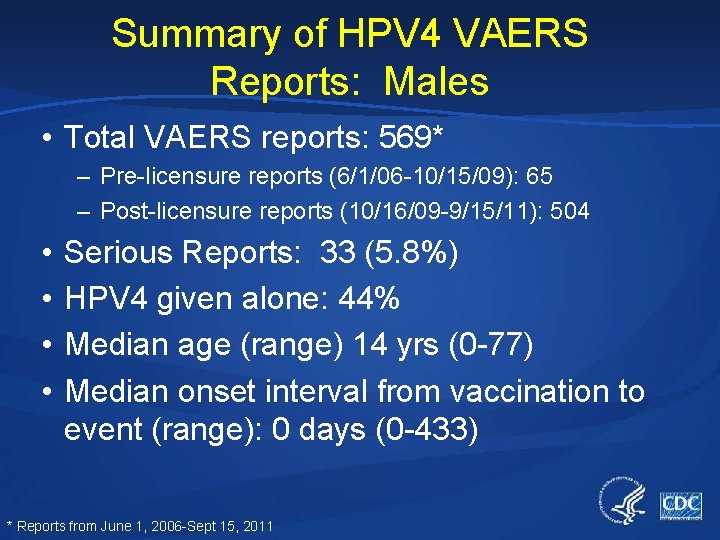
Summary of HPV 4 VAERS Reports: Males • Total VAERS reports: 569* – Pre-licensure reports (6/1/06 -10/15/09): 65 – Post-licensure reports (10/16/09 -9/15/11): 504 • • Serious Reports: 33 (5. 8%) HPV 4 given alone: 44% Median age (range) 14 yrs (0 -77) Median onset interval from vaccination to event (range): 0 days (0 -433) * Reports from June 1, 2006 -Sept 15, 2011

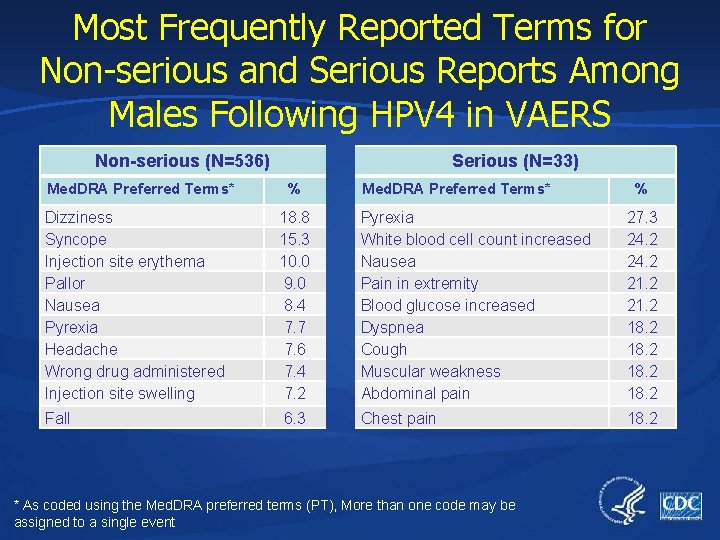
Most Frequently Reported Terms for Non-serious and Serious Reports Among Males Following HPV 4 in VAERS Non-serious (N=536) Med. DRA Preferred Terms* Serious (N=33) % Med. DRA Preferred Terms* % Dizziness Syncope Injection site erythema Pallor Nausea Pyrexia Headache Wrong drug administered Injection site swelling 18. 8 15. 3 10. 0 9. 0 8. 4 7. 7 7. 6 7. 4 7. 2 Pyrexia White blood cell count increased Nausea Pain in extremity Blood glucose increased Dyspnea Cough Muscular weakness Abdominal pain 27. 3 24. 2 21. 2 18. 2 Fall 6. 3 Chest pain 18. 2 * As coded using the Med. DRA preferred terms (PT), More than one code may be assigned to a single event

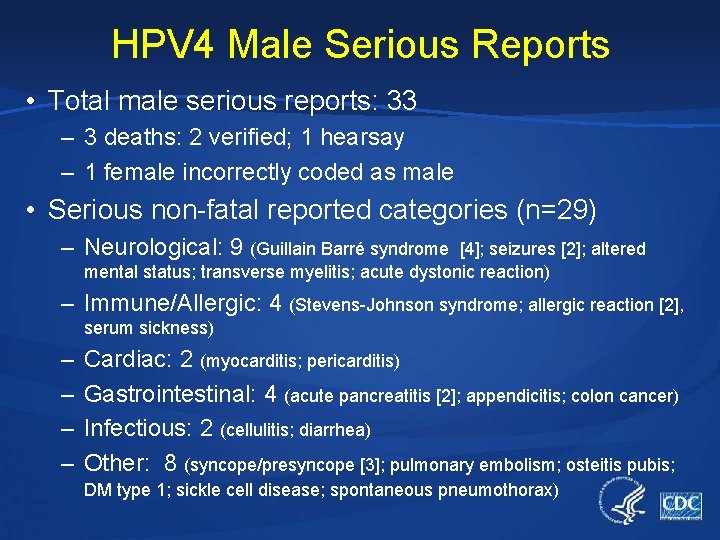
HPV 4 Male Serious Reports • Total male serious reports: 33 – 3 deaths: 2 verified; 1 hearsay – 1 female incorrectly coded as male • Serious non-fatal reported categories (n=29) – Neurological: 9 (Guillain Barré syndrome [4]; seizures [2]; altered mental status; transverse myelitis; acute dystonic reaction) – Immune/Allergic: 4 (Stevens-Johnson syndrome; allergic reaction [2], serum sickness) – – Cardiac: 2 (myocarditis; pericarditis) Gastrointestinal: 4 (acute pancreatitis [2]; appendicitis; colon cancer) Infectious: 2 (cellulitis; diarrhea) Other: 8 (syncope/presyncope [3]; pulmonary embolism; osteitis pubis; DM type 1; sickle cell disease; spontaneous pneumothorax)

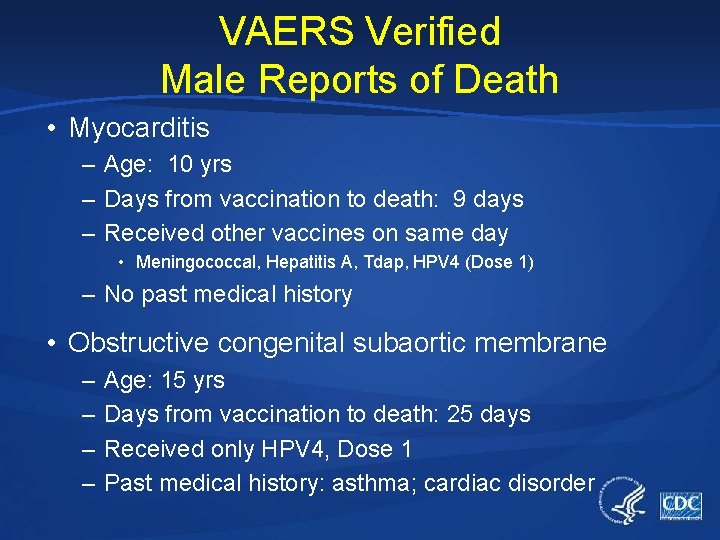
VAERS Verified Male Reports of Death • Myocarditis – Age: 10 yrs – Days from vaccination to death: 9 days – Received other vaccines on same day • Meningococcal, Hepatitis A, Tdap, HPV 4 (Dose 1) – No past medical history • Obstructive congenital subaortic membrane – – Age: 15 yrs Days from vaccination to death: 25 days Received only HPV 4, Dose 1 Past medical history: asthma; cardiac disorder

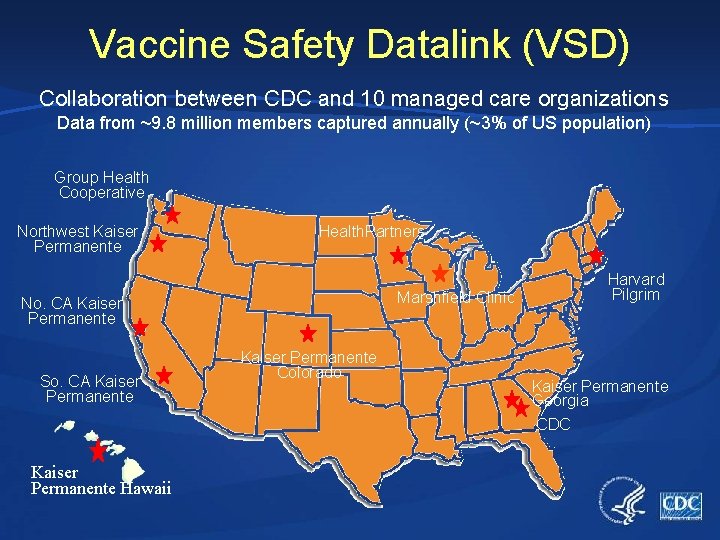
Vaccine Safety Datalink (VSD) Collaboration between CDC and 10 managed care organizations Data from ~9. 8 million members captured annually (~3% of US population) Group Health Cooperative Northwest Kaiser Permanente Health. Partners Marshfield Clinic No. CA Kaiser Permanente So. CA Kaiser Permanente Hawaii Kaiser Permanente Colorado Harvard Pilgrim Kaiser Permanente Georgia CDC

Rapid Cycle Analysis (RCA) • Alternative to traditional post-licensure vaccine safety study methods, which generally take years to complete • Basics of RCA vaccine safety monitoring: – Tests specific hypotheses with well-defined outcomes – Each week, evaluate the number of events in vaccinated persons – Compare to the expected number of events based on a comparison group • Historical or concurrent – Weekly analyses with statistical adjustment for multiple looks • RCA is not intended to be ‘final answer’ – Potential associations (“signals”) need further study to determine whether signals are real or spurious Lieu TA, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007 Oct; 45: S 89 -95.

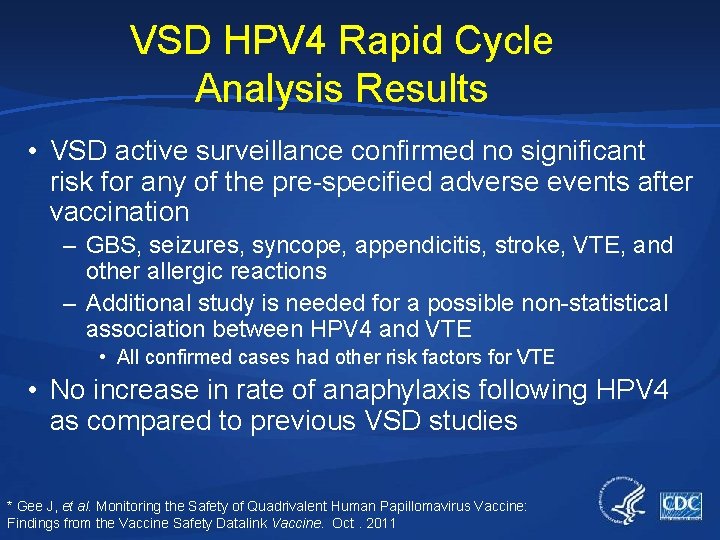
VSD HPV 4 Rapid Cycle Analysis Results • VSD active surveillance confirmed no significant risk for any of the pre-specified adverse events after vaccination – GBS, seizures, syncope, appendicitis, stroke, VTE, and other allergic reactions – Additional study is needed for a possible non-statistical association between HPV 4 and VTE • All confirmed cases had other risk factors for VTE • No increase in rate of anaphylaxis following HPV 4 as compared to previous VSD studies * Gee J, et al. Monitoring the Safety of Quadrivalent Human Papillomavirus Vaccine: Findings from the Vaccine Safety Datalink Vaccine. Oct. 2011

VSD HPV 4 Vaccine Monitoring and Evaluation: Next Steps • Long-term surveillance of GBS and stroke – Currently ongoing • VTE self-controlled case series analysis – Currently ongoing • RCA of HPV 4 in males

VSD Study: Reported Adverse Events in Young Women Following HPV 4 • Mailed survey to young women after reciept of first HPV 4 vaccine – 899 (27%) responded • Highlighted results: – Age is an important factor influencing young women’s experiences with HPV vaccine • Knowledge and attitude of HPV and HPV vaccine • Reporting of adverse events Naleway, et al. Reported Adverse Events in Young Women Following Quadivalent Human Papillomavirus Vaccination. Journal of Womens Health (In Press) 2012

Manufacturer HPV 4 Post-Licensure Commitments • Multiple studies* – A Post-Licensure Surveillance Program for the Safety of GARDASIL™ in a Managed Care Organization Setting (Protocol 31) – The Nordic long-term follow up study (Protocol 15) – A long-term immunogenicity, safety, and effectiveness study of GARDASIL™ among adolescents who received GARDASIL™ at 9 -15 yrs of age (Protocol 18) * Bonani P, et al. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine 2010

2011 Institute of Medicine (IOM) Report on Adverse Events of Vaccines • Syncope following vaccination: – IOM concluded that data convincingly support a causal relationship between injection of a vaccine and syncope • Anaphylaxis following HPV 4: – IOM concluded that the weight of published evidence favored an acceptance of a causal relationship between HPV vaccine and anaphylaxis* * http: //www. iom. edu/Reports/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality. aspx

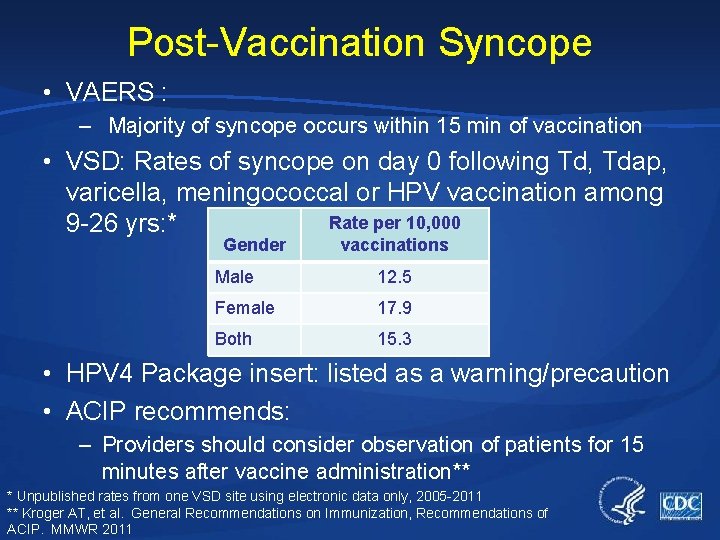
Post-Vaccination Syncope • VAERS : – Majority of syncope occurs within 15 min of vaccination • VSD: Rates of syncope on day 0 following Td, Tdap, varicella, meningococcal or HPV vaccination among Rate per 10, 000 9 -26 yrs: * Gender vaccinations Male 12. 5 Female 17. 9 Both 15. 3 • HPV 4 Package insert: listed as a warning/precaution • ACIP recommends: – Providers should consider observation of patients for 15 minutes after vaccine administration** * Unpublished rates from one VSD site using electronic data only, 2005 -2011 ** Kroger AT, et al. General Recommendations on Immunization, Recommendations of ACIP. MMWR 2011

Summary • Monitoring and evaluation are ongoing for HPV 4 • VAERS continues reviewing reports following HPV 4 – No new adverse event concerns or clinical patterns identified • VSD rapid cycle analysis confirmed no significant risk for any of the pre-specified adverse events after vaccination for two age groups (9 -17 yrs and 18 -26 yrs) – Further evaluation of VTE and HPV 4 is ongoing • Providers should consider observation of patients 15 minutes after vaccine administration

Acknowledgements Immunization Safety Office Jorge Arana Karen Broder Jonathan Duffy Theresa Harrington Zanie Leroy Paige Lewis Pedro Moro Claudia Vellozzi Eric Weintraub DSTDP Eileen Dunne Lauri Markowitz FDA David Martin Michael Nguyen Andrea Sutherland
 Hpv vaccine schedule adults
Hpv vaccine schedule adults Global vaccine safety blueprint
Global vaccine safety blueprint Vaccine safety communication
Vaccine safety communication Global vaccine safety network
Global vaccine safety network Duke human vaccine institute
Duke human vaccine institute Hpv test for men
Hpv test for men Hpv oral cancer
Hpv oral cancer Hpv cancer prevention
Hpv cancer prevention Hpv dna hr genotipleri pozitif ne demek
Hpv dna hr genotipleri pozitif ne demek Ascus hpv negativ
Ascus hpv negativ Does hpv go away
Does hpv go away Hpv tipleri
Hpv tipleri Hpv type 16 and 18
Hpv type 16 and 18 Hpv type 16 and 18
Hpv type 16 and 18 Swedescore
Swedescore Meninkok
Meninkok Citologia
Citologia Cancerscreening.gov.au
Cancerscreening.gov.au Hpv cervical cancer
Hpv cervical cancer Wanzia
Wanzia Hpv cervical cancer
Hpv cervical cancer Hpv us
Hpv us Hpv dna testi
Hpv dna testi Hpv infektion
Hpv infektion Hpv
Hpv Genital warts
Genital warts Prevention hpv
Prevention hpv Hpv
Hpv Hpv infection
Hpv infection