Kaiser Permanente Research Human Papillomavirus Vaccine Safety and

















- Slides: 28

Kaiser Permanente Research Human Papillomavirus Vaccine Safety and Effectiveness Research in an Integrated Healthcare Delivery System Allison Naleway, Ph. D Center for Health Research Kaiser Permanente Northwest Oregon HPV Summit – June 11, 2019 © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Presentation Overview • Kaiser Permanente • Center for Health Research • Vaccine Safety Research • Vaccine Effectiveness Research • Questions/Discussion © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Kaiser Permanente • Founded by Henry Kaiser and Dr. Sidney Garfield • Opened to public enrollment July 1945 • Currently provides care in 8 regions • Northern California, Southern California, Northwest, Colorado, Mid-Atlantic, Georgia, Hawaii, Washington (formerly Group Health Cooperative) • ~14 million members • All share same EMR system, Health. Connect • All regions have research capabilities © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Kaiser Permanente Northwest • ~610, 000 members in southwest Washington/northwest Oregon • Portland metropolitan area • I-5 corridor from Eugene to Longview-Kelso • ~287, 000 members also have KP dental coverage • Own 2 hospitals, network of clinics, pharmacies, clinical laboratory • Shared electronic medical record © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Center for Health. Research Kaiser Permanente § Established in 1964 § Research centers in Portland § NW has 250 employees, including 40+ investigators § $45 million budget in 2018 § 85% of funds from external grants, most from federal government © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Vaccine Research at CHR Clinical Trials Safety Effectiveness/Impact Surveillance Interventions HPV Pregnant women Varicella/Herpes zoster Influenza © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

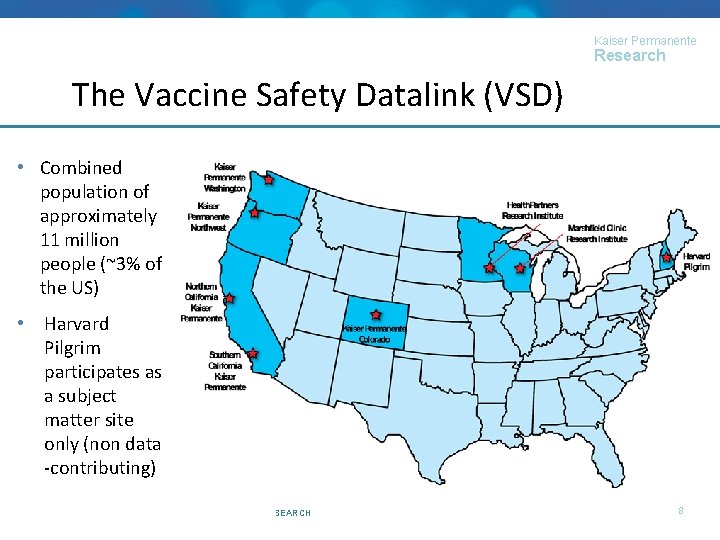
Kaiser Permanente Research The Vaccine Safety Datalink (VSD) • Collaborative project between CDC and 8 US health systems (7 data-contributing) • Established in 1990 • Allows for planned, post-licensure vaccine safety studies, as well as: • Rapid safety assessments for routine and emergency vaccination campaigns • Monitoring changes in immunization patterns following schedule changes of the introduction of new vaccines • Informs national immunization policy (e. g. , ACIP presentations and work groups) © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH 7

Kaiser Permanente Research The Vaccine Safety Datalink (VSD) • Combined population of approximately 11 million people (~3% of the US) • Harvard Pilgrim participates as a subject matter site only (non data -contributing) © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH 8

Kaiser Permanente Research Rapid Surveillance for AEs following HPV Vaccination © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Rapid Surveillance for AEs following HPV Vaccination • Monitored VSD data weekly August 2006 - October 2009 • ~600, 600 HPV doses observed in 9 -26 year old females • Evaluated 8 pre-specified possible AEs: • Guillain-Barre syndrome • Stroke • VTE • Appendicitis • Anaphylaxis • Seizures* • Syncope* © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Rapid Surveillance for AEs following HPV Vaccination • Compared incidence of these outcomes to historical background rates using max. SPRT • More common outcomes were compared to unvaccinated females with preventive care visits • No increased risk of any outcome was observed • VTE RR was 1. 98 (not statistically significant) in 9 -17 year olds © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research HPV Vaccination and VTE © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

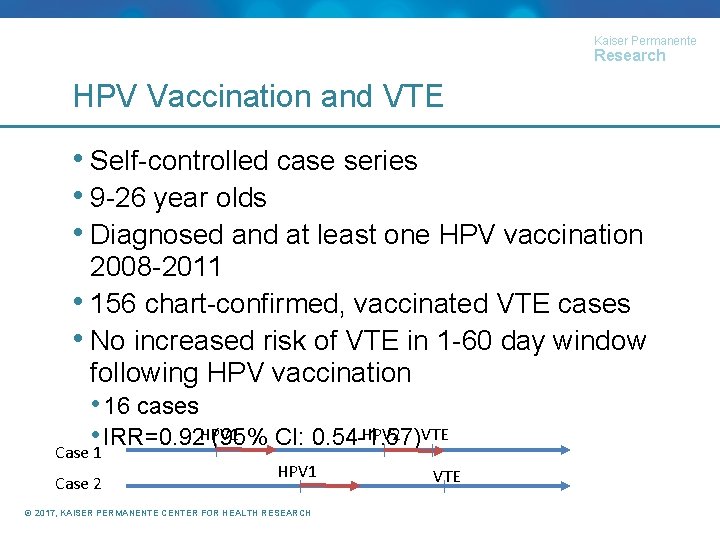
Kaiser Permanente Research HPV Vaccination and VTE • Self-controlled case series • 9 -26 year olds • Diagnosed and at least one HPV vaccination 2008 -2011 • 156 chart-confirmed, vaccinated VTE cases • No increased risk of VTE in 1 -60 day window following HPV vaccination • 16 cases HPV 2 VTE • IRR=0. 92 HPV 1 (95% CI: 0. 54 -1. 57) Case 1 Case 2 HPV 1 © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH VTE

Kaiser Permanente Research VIVE Study © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research VIVE Study • Surveyed 899 young women (11 -26 years of age) in 2008 within a few days of receiving first HPV dose • 78% reported pain at injection site • 17% bruising or discoloration at injection site • 14% swelling at injection site • 15% syncope or pre-syncope • These are commonly reported vaccine reactions • Syncope is especially an issue when © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research POI and Adolescent Vaccination © 2017, KAISER FOR HEALTH RESEARCHPMID 30131438 Naleway AL, et. PERMANENTE al. Pediatrics. CENTER 2018; 142(3): pii: e 20180943. 16

Kaiser Permanente Research POI and Adolescent Vaccination Background • Characterized by dysfunction or depletion of ovarian follicles, menopausal symptoms, and reduced fertility • Typically idiopathic, but may be associated with underlying autoimmune or infectious disease • Recent public concerns of a possible association between POI and HPV vaccine - stemming from published case series, media attention, and social media/internet content © 2017, KAISER FOR HEALTH RESEARCHPMID 30131438 Naleway AL, et. PERMANENTE al. Pediatrics. CENTER 2018; 142(3): pii: e 20180943. 17

Kaiser Permanente Research POI and Adolescent Vaccination Study Objectives • Identify and describe characteristics of idiopathic POI diagnosed in females aged 11 -34 years • Describe prevalence and age-specific incidence of idiopathic POI • Estimate the risk of idiopathic POI in females following HPV vaccination and other adolescent vaccinations © 2017, KAISER FOR HEALTH RESEARCHPMID 30131438 Naleway AL, et. PERMANENTE al. Pediatrics. CENTER 2018; 142(3): pii: e 20180943. 18

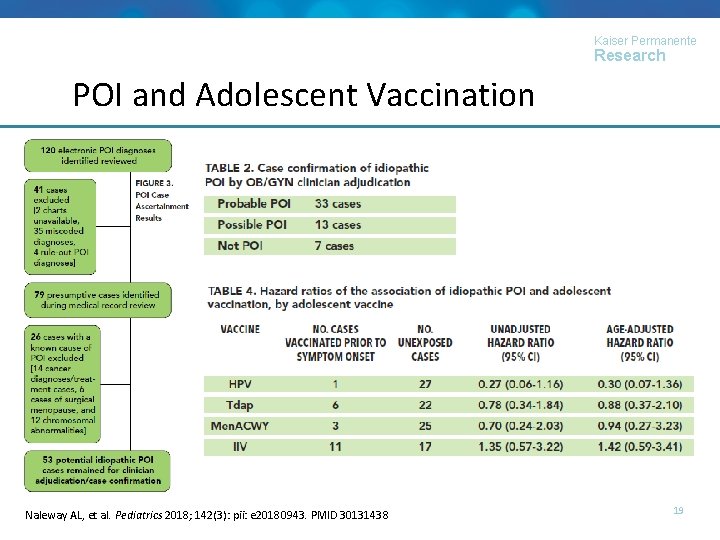
Kaiser Permanente Research POI and Adolescent Vaccination © 2017, KAISER FOR HEALTH RESEARCHPMID 30131438 Naleway AL, et. PERMANENTE al. Pediatrics. CENTER 2018; 142(3): pii: e 20180943. 19

Kaiser Permanente Research POI and Adolescent Vaccination Challenges • Time from symptom onset to diagnosis may be variable or long (median = 3 years); onset difficult to ascertain when cases present with primary amenorrhea • Diagnoses of POI may be difficult to accurately identify due to variability of testing practices within health care systems Conclusion • No evidence of increased risk of idiopathic POI was identified following HPV vaccine exposure or other routine adolescent vaccination Naleway AL, et al. Pediatrics 2018; 142(3): pii: e 20180943. PMID 30131438 © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH 20

Kaiser Permanente Research Other HPV Vaccine Safety Studies • In Process • Complex Regional Pain Syndrome (CRPS) • Postural Orthostatic Tachycardia Syndrome (POTS) • Chronic Fatigue Syndrome (CFS)/Myalgic Encephalomyelitis (ME) • Syncope and Syncope-related Injuries • Completed • Rapid Safety Monitoring of 9 v. HPV • Safety of Inadvertent HPV Vaccine Exposure during Pregnancy • HPV Vaccination and Autoimmune Disease Diagnoses © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Measuring HPV Vaccine Effectiveness • 20 -30 years between HPV exposure and cervical cancer diagnosis • Need intermediary outcomes to evaluate effectiveness • HPV infection • Anogenital warts • Cervical cancer precursor lesions © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research HPV Vaccine Impact Study (Hi. TS) © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Hi. TS (paper currently under review) • HPV prevalence in Pap test specimens • Collection periods: 2007, 2012 -13, 2015 -16 • CDC lab performed HPV testing • VT-HPV prevalence (6, 11, 16, 18) decreased • 78% in 20 -24 year old females • 38% in 25 -29 year old females • Decreases observed in vaccinated and unvaccinated suggesting both direct and herd protection © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

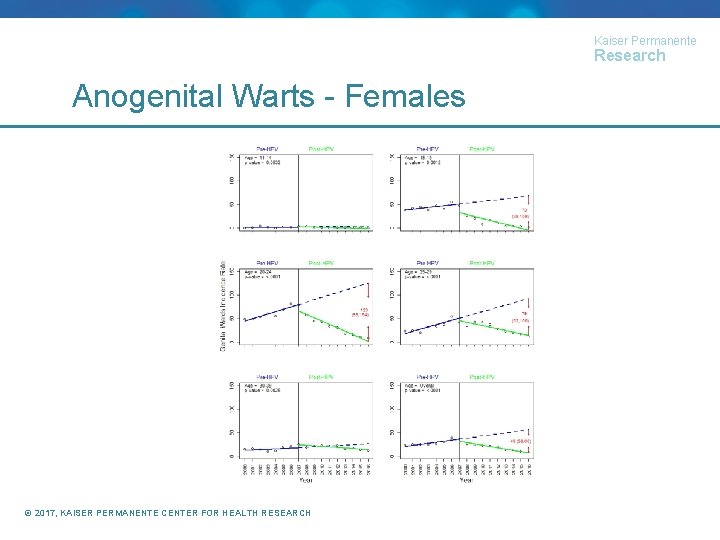
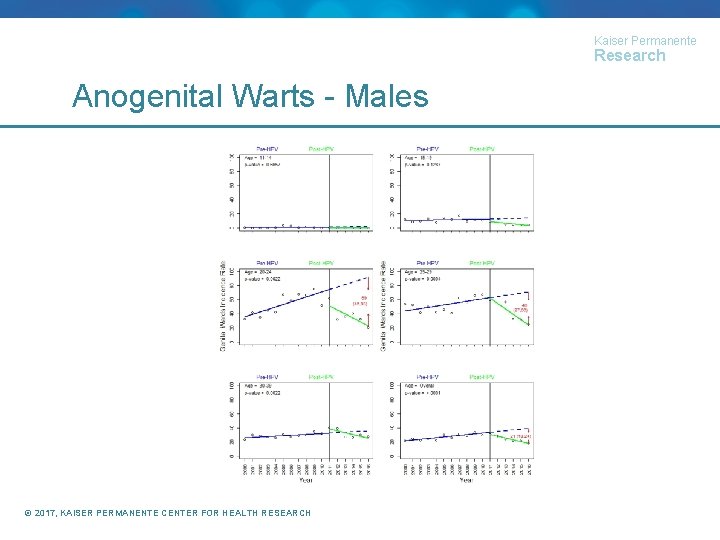
Kaiser Permanente Research Anogenital Wart Incidence (paper under review) • Identified members 11 -39 years of age with at least one calendar year of enrollment • Calculated AGW incidence by age group, calendar year, and gender • Conducted interrupted time series using segmented regression to compare AGW incidence in the periods before and after HPV recommendations • Females: 2000 -2006 vs 2007 -2016 • Males: 2000 -2010 vs 2011 -2016 © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Anogenital Warts - Females © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Anogenital Warts - Males © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH

Kaiser Permanente Research Conclusions • HPV vaccine is safe and effective • The data and resources available to us at KP allow us to address important and wide-ranging topics in immunization safety, effectiveness, and delivery • Thank you! • Questions? Discussion? © 2017, KAISER PERMANENTE CENTER FOR HEALTH RESEARCH
 Kaiser vision statement
Kaiser vision statement Code purple kaiser
Code purple kaiser Kaiser hospital santa cruz
Kaiser hospital santa cruz Kaiser permanente pharmacy eugene
Kaiser permanente pharmacy eugene Kaiser bridge program
Kaiser bridge program Kaiser permanente community benefit program
Kaiser permanente community benefit program Rx bin kaiser
Rx bin kaiser Kaiser permanente value compass
Kaiser permanente value compass Kaisermyhealth
Kaisermyhealth Pamela schwartz
Pamela schwartz Kaiser san diego family medicine residency
Kaiser san diego family medicine residency Kaiser permanente wa pharmacy
Kaiser permanente wa pharmacy Global vaccine safety blueprint
Global vaccine safety blueprint Vaccine safety communication
Vaccine safety communication Global vaccine safety network
Global vaccine safety network Duke human vaccine institute
Duke human vaccine institute Vaccine storage and handling sop worksheet
Vaccine storage and handling sop worksheet Vaccine storage and handling protocol
Vaccine storage and handling protocol Definition of vaccine
Definition of vaccine Edible vaccines pros and cons
Edible vaccines pros and cons Cats rabies
Cats rabies Bactivor
Bactivor Hep b vaccine schedule for adults
Hep b vaccine schedule for adults Zostavax reconstitution
Zostavax reconstitution Grits ga registry of immunization
Grits ga registry of immunization Hpv vaccine schedule adults
Hpv vaccine schedule adults Alternative vaccine schedule 2021
Alternative vaccine schedule 2021 Next generation vaccine
Next generation vaccine Mcv4 vaccine schedule
Mcv4 vaccine schedule