GAVI Vaccine Investment Strategy Human Papillomavirus Analysis Final
























- Slides: 40

GAVI Vaccine Investment Strategy Human Papillomavirus Analysis Final October 27, 2008

HPV Human Papillomavirus (HPV) CONTENTS • Disease Overview • Vaccine Landscape • Vaccination Policy & Strategies • Vaccine Need & Adoption Forecast • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 2

HPV Disease Overview DISEASE PATHOGEN, TRANSMISSION & TARGET POPULATION 1 -3 • • Human Papillomavirus (HPV) infection is responsible for >95% of cervical cancers worldwide – Anal, vulvar/penile, and head & neck cancers are also associated with HPV infection – > 100 HPV strains are known • HPV-16 & -18 infections can lead to cervical cancer and high-grade cervical intraepithelial neoplasia • HPV-6 and -11 are mainly associated with genital warts Transmission – • • Infection is spread through sexual contact Geographic Distribution – Both HPV-16 & -18 linked cervical cancer have been reported worldwide and account for ~70% of the cases (~52% for high-grade cervical intraepithelial neoplasia) – HPV-6 & -11 are rarely associated with cancer, but cause >90% of genital warts – Regional HPV strain variance exists with HPV-45 & -33 also found in Africa, -45 & -52 in S. Asia, and -45 & -31 in the Americas and Europe Disease Target Population – Peak of HPV infection coincides with the onset of sexual activity in girls and young women 3

HPV Disease Overview DISEASE IMPACT 4 -6 • Total Morbidity – • Total Mortality – • Over half of the women diagnosed with cervical cancer die from the disease (~260, 000), and 85% of those deaths occur in the developing world where cancer screening is uncommon Epidemic Potential – • ~500, 000 women are diagnosed with cervical cancer each year, about 80% of which are in the developing countries Endemic disease Disease Sequelae – Girls and young women are generally infected with HPV when they become sexually active, but it can take 20 years or longer for infections to progress to cervical cancer – ~5% to 10% of women infected with oncogenic HPV ultimately develop persistent infections that can lead to precancerous lesions that can develop into cervical cancer – In the industrialized countries, the five-year survival rate is ~70%, it’s expected to be much lower in the developing countries – Anal cancer is of growing concern, especially among men who have sex with men, because HIV infection increases susceptibility to HPV-related diseases of all kinds 4

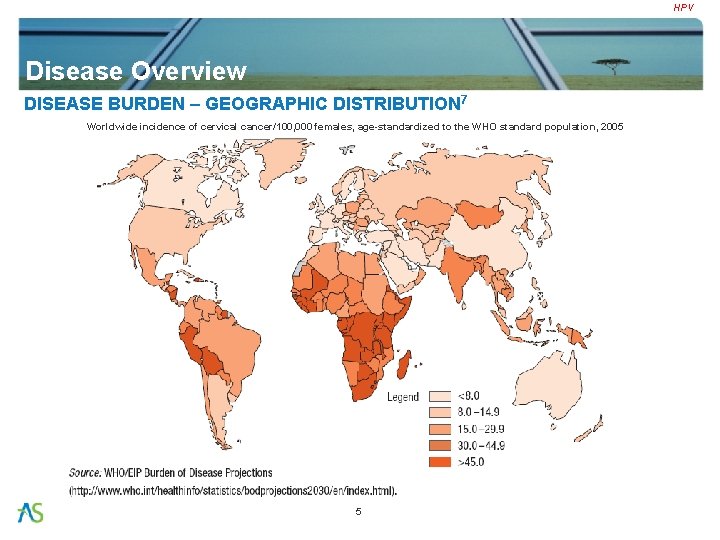
HPV Disease Overview DISEASE BURDEN – GEOGRAPHIC DISTRIBUTION 7 Worldwide incidence of cervical cancer/100, 000 females, age-standardized to the WHO standard population, 2005 5

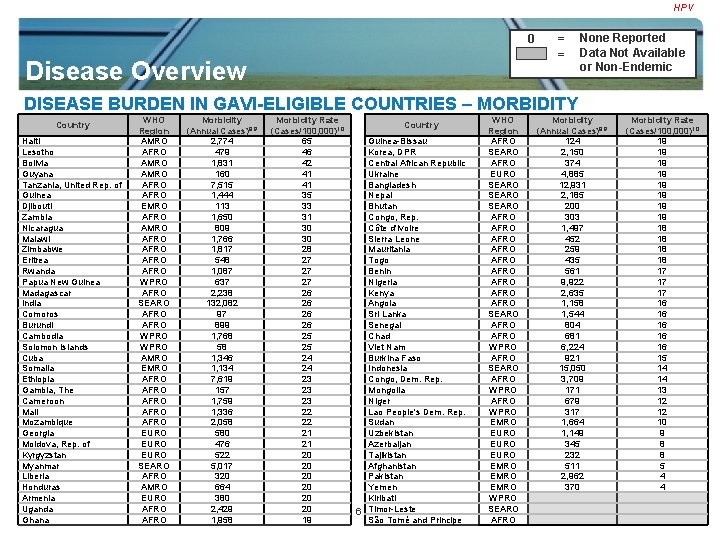
HPV 0 Disease Overview = = None Reported Data Not Available or Non-Endemic DISEASE BURDEN IN GAVI-ELIGIBLE COUNTRIES – MORBIDITY WHO Morbidity Region (Annual Cases)8, 9 Haiti AMRO 2, 774 Lesotho AFRO 479 Bolivia AMRO 1, 831 Guyana AMRO 160 Tanzania, United Rep. of AFRO 7, 515 Guinea AFRO 1, 444 Djibouti EMRO 113 Zambia AFRO 1, 650 Nicaragua AMRO 809 Malawi AFRO 1, 766 Zimbabwe AFRO 1, 817 Eritrea AFRO 548 Rwanda AFRO 1, 087 Papua New Guinea WPRO 637 Madagascar AFRO 2, 238 India SEARO 132, 082 Comoros AFRO 97 Burundi AFRO 899 Cambodia WPRO 1, 768 Solomon Islands WPRO 58 Cuba AMRO 1, 346 Somalia EMRO 1, 134 Ethiopia AFRO 7, 619 Gambia, The AFRO 157 Cameroon AFRO 1, 759 Mali AFRO 1, 336 Mozambique AFRO 2, 058 Georgia EURO 580 Moldova, Rep. of EURO 476 Kyrgyzstan EURO 522 Myanmar SEARO 5, 017 Liberia AFRO 320 Honduras AMRO 664 Armenia EURO 380 Uganda GAVI Vaccine Investment AFROStrategy 2, 429 Ghana Vaccine Landscape Analysis_Cholera_Apr 08 AFRO 1, 958 Country Morbidity Rate (Cases/100, 000)10 65 46 42 41 41 35 33 31 30 30 28 27 27 27 26 26 25 25 24 24 23 23 23 22 22 21 21 20 20 20 19 Country Guinea-Bissau Korea, DPR Central African Republic Ukraine Bangladesh Nepal Bhutan Congo, Rep. Côte d'Ivoire Sierra Leone Mauritania Togo Benin Nigeria Kenya Angola Sri Lanka Senegal Chad Viet Nam Burkina Faso Indonesia Congo, Dem. Rep. Mongolia Niger Lao People's Dem. Rep. Sudan Uzbekistan Azerbaijan Tajikistan Afghanistan Pakistan 4 Yemen Kiribati 6 Timor-Leste São Tomé and Principe WHO Region AFRO SEARO AFRO EURO SEARO AFRO AFRO AFRO SEARO AFRO WPRO EMRO EURO EMRO WPRO SEARO AFRO Morbidity (Annual Cases)8, 9 124 2, 150 374 4, 885 12, 931 2, 185 200 303 1, 497 452 259 435 561 9, 922 2, 635 1, 158 1, 544 804 681 6, 224 921 15, 050 3, 709 171 679 317 1, 664 1, 149 345 232 511 2, 962 370 Morbidity Rate (Cases/100, 000)10 19 19 18 18 17 17 17 16 16 16 15 14 14 13 12 12 10 9 8 8 5 4 4

HPV 0 Disease Overview = = None Reported Data Not Available or Non-Endemic DISEASE BURDEN IN GAVI-ELIGIBLE COUNTRIES – MORTALITY WHO Mortality Rate Country Region (Annual Deaths)8, 11 (Deaths/1, 000)12 Lesotho AFRO 391 376 Benin Haiti AMRO 1, 484 346 Angola Tanzania, United Rep. of AFRO 6, 009 325 Kenya Guinea AFRO 1, 138 273 Nicaragua Djibouti EMRO 90 268 Chad Zambia AFRO 1, 340 248 Senegal Malawi AFRO 1, 405 237 Burkina Faso Zimbabwe AFRO 1, 492 229 Congo, Dem. Rep. Bolivia AMRO 987 226 Honduras Eritrea AFRO 438 219 Myanmar Rwanda AFRO 878 219 Cuba Madagascar AFRO 1, 795 211 Moldova, Rep. of Burundi AFRO 722 209 Ukraine Comoros AFRO 79 209 Bhutan Somalia EMRO 906 185 Nepal Ethiopia AFRO 6, 081 183 Bangladesh Cameroon AFRO 1, 419 182 Niger Guyana AMRO 71 182 Sri Lanka Gambia, The AFRO 124 179 Sudan Mali AFRO 1, 076 178 Georgia Mozambique AFRO 1, 654 173 Viet Nam Liberia AFRO 256 159 Kyrgyzstan Guinea-Bissau AFRO 99 157 Mongolia Central African Republic AFRO 306 155 Indonesia Ghana AFRO 1, 572 155 Armenia Uganda AFRO 1, 932 155 Lao People's Dem. Rep. Congo, Rep. AFRO 242 148 Korea, DPR Sierra Leone AFRO 362 148 Uzbekistan Côte d'Ivoire AFRO 1, 192 147 Azerbaijan India SEARO 74, 118 147 Tajikistan Mauritania AFRO 209 146 Afghanistan Togo AFRO 349 146 Pakistan 4 Yemen Papua New Guinea WPRO 341 142 Cambodia WPRO 949 135 São Tomé and Principe GAVI Vaccine Investment Nigeria AFRO Strategy 8, 030 135 7 Kiribati Vaccine Landscape. WPRO Analysis_Cholera_Apr 08 Solomon Islands 31 135 Timor-Leste Country WHO Region AFRO AFRO AMRO SEARO AMRO EURO SEARO AFRO SEARO EMRO EURO WPRO SEARO EURO EMRO AFRO WPRO SEARO Mortality Rate (Annual Deaths)8, 11 (Deaths/1, 000)12 448 133 926 132 2, 111 132 354 132 555 131 640 129 724 115 3, 058 111 361 108 2, 594 106 567 101 220 99 2, 578 99 105 96 1, 129 96 6, 561 94 532 92 840 89 1, 354 84 225 83 3, 334 83 186 73 92 71 7, 566 70 130 67 159 57 558 50 379 29 113 28 70 23 254 22 1, 605 22 206 21

HPV Disease Overview NON-VACCINE PREVENTION & TREATMENT INTERVENTIONS 13 -15 • Non-Vaccine Preventions – Condoms – Early detection/Screening/and Pre-cancer treatment – Supplements • • Studies indicate that some supplements and higher levels of fruit and vegetable consumption may decrease the risk of HPV persistence (e. g. vitamin C, E, and carotenoids) Treatment Interventions – Currently, no drug treatment for HPV infection; potential drugs are currently in development – Following options are available for treating invasive cancer: • Surgery • Radiation Therapy • Chemotherapy 8

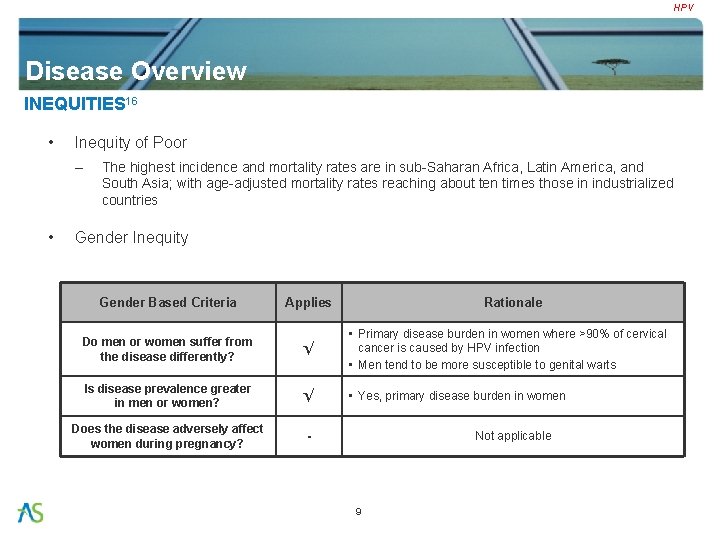
HPV Disease Overview INEQUITIES 16 • Inequity of Poor – • The highest incidence and mortality rates are in sub-Saharan Africa, Latin America, and South Asia; with age-adjusted mortality rates reaching about ten times those in industrialized countries Gender Inequity Gender Based Criteria Applies Rationale Do men or women suffer from the disease differently? √ • Primary disease burden in women where >90% of cervical cancer is caused by HPV infection • Men tend to be more susceptible to genital warts Is disease prevalence greater in men or women? √ • Yes, primary disease burden in women Does the disease adversely affect women during pregnancy? - Not applicable 9

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccination Policy & Strategies • Vaccine Need & Adoption Forecast • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 10

HPV Vaccine Landscape LICENSED VACCINES 17 Supplier (1° Partner) Merck 18 -21 GSK 21 -23 Vaccine Gardasil Cervarix Strain / Antigen HPV-6/L 1 HPV-11/L 1 HPV-16/L 1 HPV-18/L 1 Adjuvant / Platform Amorphous Al(OH)(PO 4) sulfate / VLP AS 04 (MPL, Al(OH)3) / VLP Administration Route IM IM Formulation Liquid Presentation 5 dose vials & pre-filled syringes 2 dose vials & pre-filled syringes Dosing Schedule 0, 2, 6 mo 0, 1, 6 mo Target Population for Licensure Females ≥ 9 yo Females ≥ 10 yo Safety No major safety concerns Efficacy 100% against HPV-6, 11, 16 & 18 100% against HPV-16 & 18 Expected Duration of Protection At least 5 yrs, to date At least 6. 4 yrs, to date Licensure Date (Location) Jun 06 (US) Sep 06 (EU) Sep 07 (EU) ~2009 (US) Estimated WHO PQ Date 4 Q 08 (submitted in May 07) 1 Q 09 (submitted in Sept 07) 11

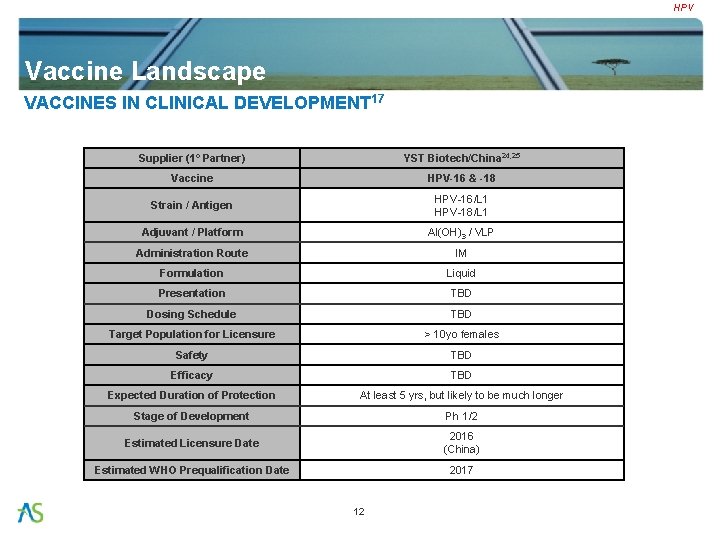
HPV Vaccine Landscape VACCINES IN CLINICAL DEVELOPMENT 17 Supplier (1° Partner) YST Biotech/China 24, 25 Vaccine HPV-16 & -18 Strain / Antigen HPV-16/L 1 HPV-18/L 1 Adjuvant / Platform Al(OH)3 / VLP Administration Route IM Formulation Liquid Presentation TBD Dosing Schedule TBD Target Population for Licensure > 10 yo females Safety TBD Efficacy TBD Expected Duration of Protection At least 5 yrs, but likely to be much longer Stage of Development Ph 1/2 Estimated Licensure Date 2016 (China) Estimated WHO Prequalification Date 2017 12

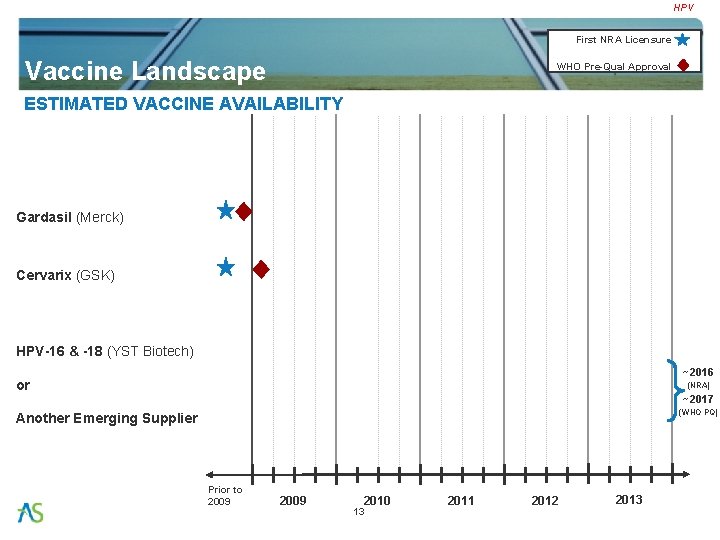
HPV First NRA Licensure Vaccine Landscape WHO Pre-Qual Approval ESTIMATED VACCINE AVAILABILITY Gardasil (Merck) Cervarix (GSK) HPV-16 & -18 (YST Biotech) ~2016 or (NRA) ~2017 (WHO PQ) Another Emerging Supplier Prior to 2009 2010 13 2011 2012 2013

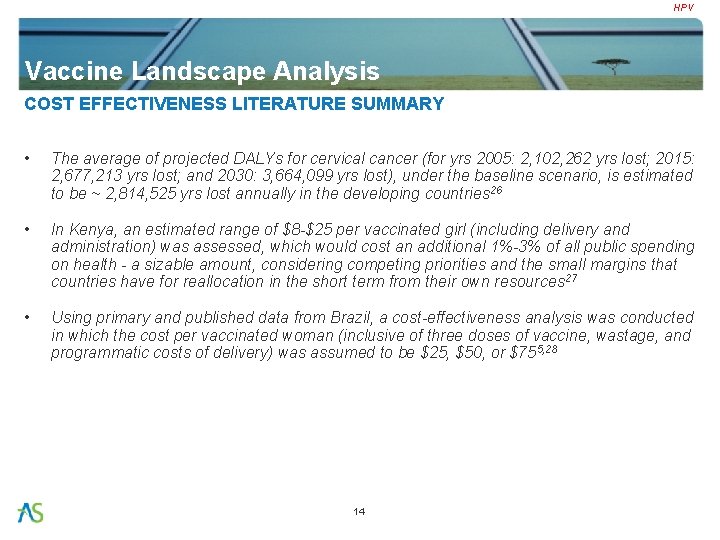
HPV Vaccine Landscape Analysis COST EFFECTIVENESS LITERATURE SUMMARY • The average of projected DALYs for cervical cancer (for yrs 2005: 2, 102, 262 yrs lost; 2015: 2, 677, 213 yrs lost; and 2030: 3, 664, 099 yrs lost), under the baseline scenario, is estimated to be ~ 2, 814, 525 yrs lost annually in the developing countries 26 • In Kenya, an estimated range of $8 -$25 per vaccinated girl (including delivery and administration) was assessed, which would cost an additional 1%-3% of all public spending on health - a sizable amount, considering competing priorities and the small margins that countries have for reallocation in the short term from their own resources 27 • Using primary and published data from Brazil, a cost-effectiveness analysis was conducted in which the cost per vaccinated woman (inclusive of three doses of vaccine, wastage, and programmatic costs of delivery) was assumed to be $25, $50, or $755, 28 14

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccination Policy & Strategies • Vaccine Need & Adoption Forecast • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 15

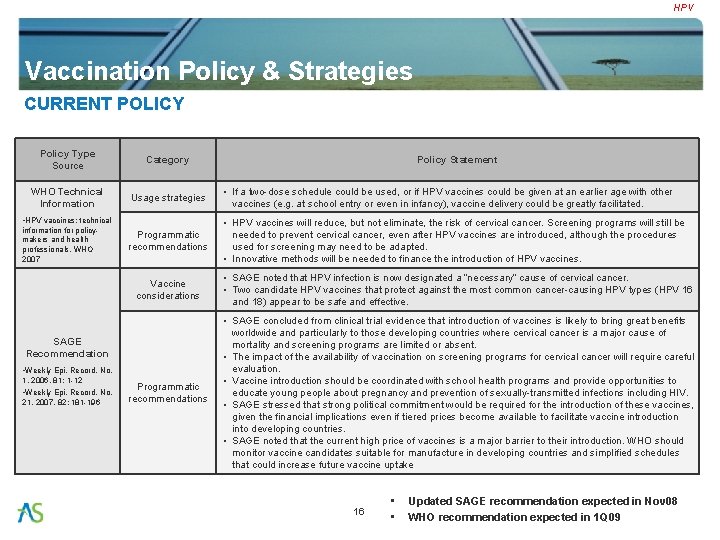
HPV Vaccination Policy & Strategies CURRENT POLICY Policy Type Source Category WHO Technical Information Usage strategies • If a two-dose schedule could be used, or if HPV vaccines could be given at an earlier age with other vaccines (e. g. at school entry or even in infancy), vaccine delivery could be greatly facilitated. Programmatic recommendations • HPV vaccines will reduce, but not eliminate, the risk of cervical cancer. Screening programs will still be needed to prevent cervical cancer, even after HPV vaccines are introduced, although the procedures used for screening may need to be adapted. • Innovative methods will be needed to finance the introduction of HPV vaccines. • HPV vaccines: technical information for policymakers and health professionals, WHO 2007 Vaccine considerations • SAGE noted that HPV infection is now designated a “necessary” cause of cervical cancer. • Two candidate HPV vaccines that protect against the most common cancer-causing HPV types (HPV 16 and 18) appear to be safe and effective. Programmatic recommendations • SAGE concluded from clinical trial evidence that introduction of vaccines is likely to bring great benefits worldwide and particularly to those developing countries where cervical cancer is a major cause of mortality and screening programs are limited or absent. • The impact of the availability of vaccination on screening programs for cervical cancer will require careful evaluation. • Vaccine introduction should be coordinated with school health programs and provide opportunities to educate young people about pregnancy and prevention of sexually-transmitted infections including HIV. • SAGE stressed that strong political commitment would be required for the introduction of these vaccines, given the financial implications even if tiered prices become available to facilitate vaccine introduction into developing countries. • SAGE noted that the current high price of vaccines is a major barrier to their introduction. WHO should monitor vaccine candidates suitable for manufacture in developing countries and simplified schedules that could increase future vaccine uptake SAGE Recommendation • Weekly Epi. Record, No. 1, 2006, 81: 1 -12 • Weekly Epi. Record, No. 21, 2007, 82: 181 -196 Policy Statement 16 • • Updated SAGE recommendation expected in Nov 08 WHO recommendation expected in 1 Q 09

HPV Vaccination Policy & Strategies VISP DECISION FRAMEWORK Vaccination Strategies for Financial Planning Purposes GAVI VIS Decision Framework Routine Vx of ~10 yo females Offer Vaccine Financing to GAVI-Eligible Countries Do Not Support in 2009 - 2013 17

HPV CONTENTS • Vaccination Policy & Strategies • Disease Overview • Vaccine Landscape • Vaccine Need & Adoption Forecast • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 18

HPV Vaccine Need & Adoption Forecast GAVI-ELIGIBLE COUNTRY VACCINE NEED Vaccine Need: 72 VISP Scope: 60 • Cervical cancer is the most common cancer affecting women in developing countries, 5 12 countries not expected to adopt by 2020 (PATH HPV Team) GAVI-Eligible Country Vaccine Need Category (# out of 72) AFRO (34 of 36) AMRO (6 of 6) Least Poor (10 of 12) Cameroon Bolivia Guyana Honduras Intermediate (12 of 16) Ghana Kenya Cuba Nicaragua Poorest (29 of 31) Fragile (9 of 13) Zimbabwe Senegal Sierra Leone Tanzania Togo Uganda Zambia Guinea-Bissau Lesotho Madagascar Malawi Mali Mauritania Mozambique Niger Rwanda Angola Burundi CAR Congo DR Congo Côte d'Ivoire Eritrea Liberia Benin Burkina Faso Chad Comoros Ethiopia Gambia Guinea ORANGE: countries expecting to adopt after 2020 Haiti 19 EMRO (6 of 6) EURO (8 of 8) SEARO (9 of 9) WPRO (6 of 7) Djibouti Armenia Azerbaijan Georgia Ukraine Indonesia Sri Lanka Kiribati Pakistan Kyrgyzstan Moldova Tajikistan Uzbekistan India Korea DPR Mongolia PNG Viet Nam Yemen Bangladesh Bhutan Myanmar Nepal Cambodia Lao Solomon Islands Afghanistan Somalia Sudan Timor-Leste Source: WHO & PATH HPV Team

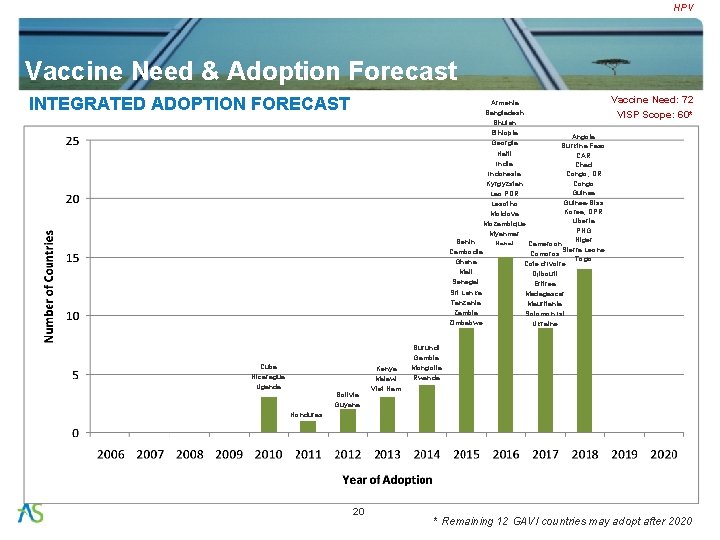
HPV Vaccine Need & Adoption Forecast INTEGRATED ADOPTION FORECAST Armenia Bangladesh Bhutan Ethiopia Angola Georgia Burkina Faso Haiti CAR India Chad Indonesia Congo, DR Congo Kyrgyzstan Guinea Lao PDR Guinea-Biss Lesotho Korea, DPR Moldova Liberia Mozambique PNG Myanmar Niger Benin Cameroon Nepal Sierra Leone Cambodia Comoros Togo Ghana Cote d’Ivoire Mali Djibouti Senegal Eritrea Sri Lanka Madagascar Tanzania Mauritania Zambia Solomon Isl Zimbabwe Ukraine Cuba Nicaragua Uganda Bolivia Guyana Kenya Malawi Viet Nam Vaccine Need: 72 VISP Scope: 60* Burundi Gambia Mongolia Rwanda Honduras 20 * Remaining 12 GAVI countries may adopt after 2020

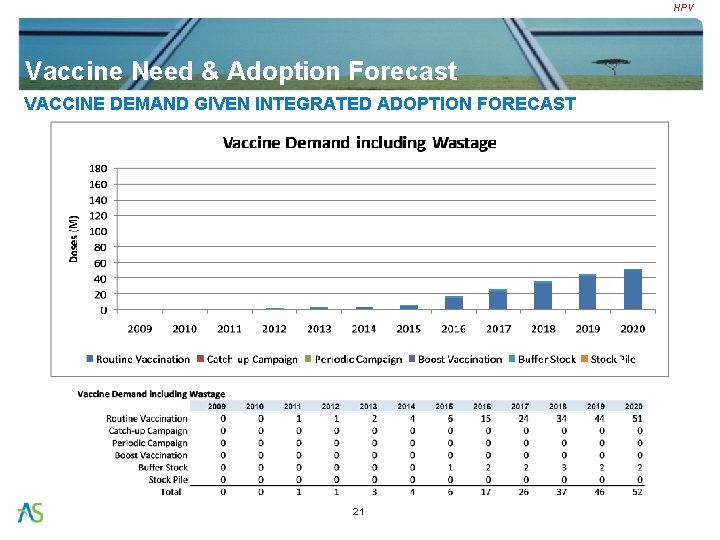
HPV Vaccine Need & Adoption Forecast VACCINE DEMAND GIVEN INTEGRATED ADOPTION FORECAST 21

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccination Policy & Strategies • Vaccine Need & Adoption Forecast • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 22

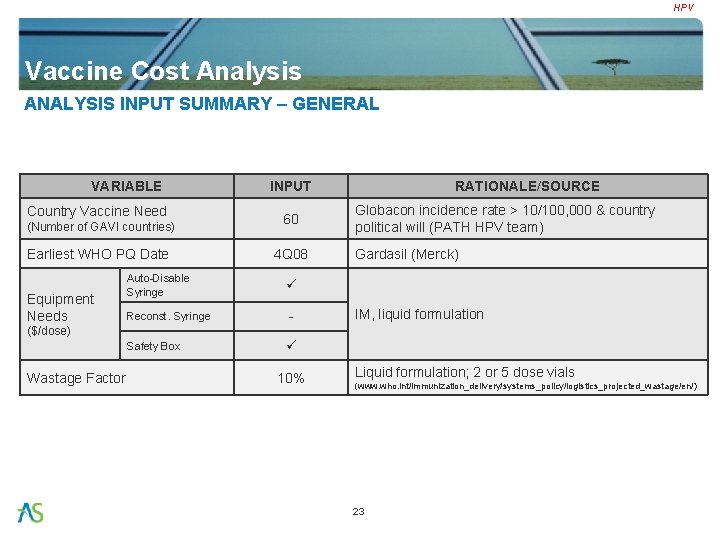
HPV Vaccine Cost Analysis ANALYSIS INPUT SUMMARY – GENERAL VARIABLE Country Vaccine Need (Number of GAVI countries) Earliest WHO PQ Date Equipment Needs INPUT 60 4 Q 08 Auto-Disable Syringe Reconst. Syringe - Safety Box RATIONALE/SOURCE Globacon incidence rate > 10/100, 000 & country political will (PATH HPV team) Gardasil (Merck) IM, liquid formulation ($/dose) Wastage Factor 10% Liquid formulation; 2 or 5 dose vials (www. who. int/immunization_delivery/systems_policy/logistics_projected_wastage/en/) 23

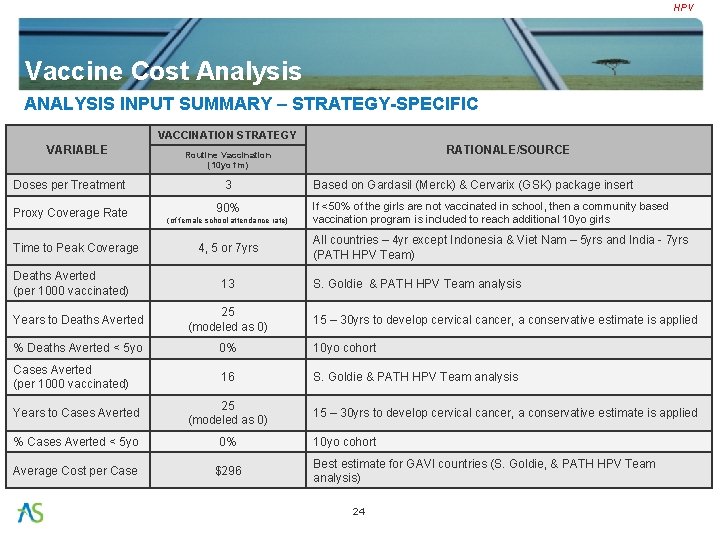
HPV Vaccine Cost Analysis ANALYSIS INPUT SUMMARY – STRATEGY-SPECIFIC VACCINATION STRATEGY VARIABLE Doses per Treatment Proxy Coverage Rate Time to Peak Coverage Deaths Averted (per 1000 vaccinated) RATIONALE/SOURCE Routine Vaccination (10 yo fm) 3 90% (of female school attendance rate) 4, 5 or 7 yrs 13 Based on Gardasil (Merck) & Cervarix (GSK) package insert If <50% of the girls are not vaccinated in school, then a community based vaccination program is included to reach additional 10 yo girls All countries – 4 yr except Indonesia & Viet Nam – 5 yrs and India - 7 yrs (PATH HPV Team) S. Goldie & PATH HPV Team analysis Years to Deaths Averted 25 (modeled as 0) % Deaths Averted < 5 yo 0% 10 yo cohort Cases Averted (per 1000 vaccinated) 16 S. Goldie & PATH HPV Team analysis Years to Cases Averted 25 (modeled as 0) % Cases Averted < 5 yo 0% Average Cost per Case $296 15 – 30 yrs to develop cervical cancer, a conservative estimate is applied 10 yo cohort Best estimate for GAVI countries (S. Goldie, & PATH HPV Team analysis) 24

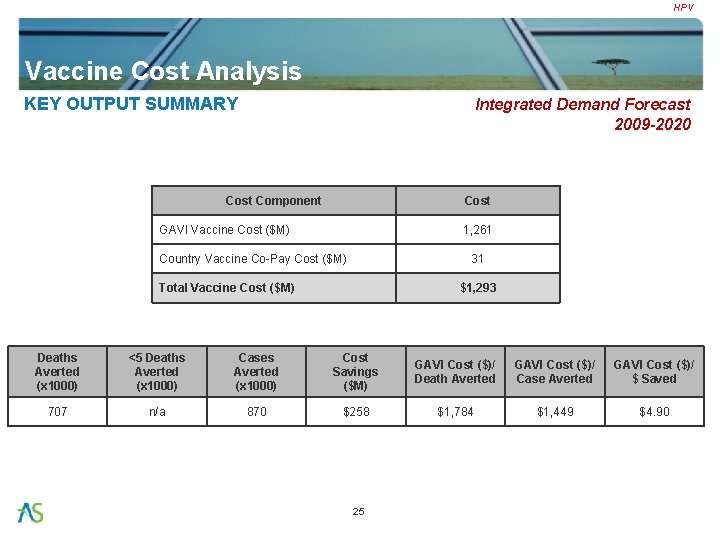
HPV Vaccine Cost Analysis KEY OUTPUT SUMMARY Integrated Demand Forecast 2009 -2020 Cost Component Cost GAVI Vaccine Cost ($M) 1, 261 Country Vaccine Co-Pay Cost ($M) 31 Total Vaccine Cost ($M) $1, 293 Deaths Averted (x 1000) <5 Deaths Averted (x 1000) Cases Averted (x 1000) Cost Savings ($M) GAVI Cost ($)/ Death Averted GAVI Cost ($)/ Case Averted GAVI Cost ($)/ $ Saved 707 n/a 870 $258 $1, 784 $1, 449 $4. 90 25

HPV Vaccine Cost Analysis ANNUAL ANALYSIS RESULTS Integrated Demand Forecast 2009 -2020 26

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccine Need & Adoption Forecast • Vaccination Policy & Strategies • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 27

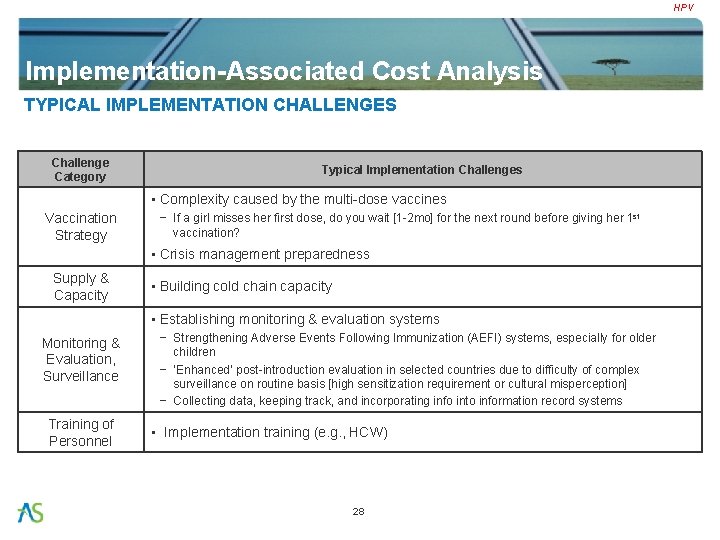
HPV Implementation-Associated Cost Analysis TYPICAL IMPLEMENTATION CHALLENGES Challenge Category Typical Implementation Challenges • Complexity caused by the multi-dose vaccines Vaccination Strategy − If a girl misses her first dose, do you wait [1 -2 mo] for the next round before giving her 1 st vaccination? • Crisis management preparedness Supply & Capacity • Building cold chain capacity • Establishing monitoring & evaluation systems Monitoring & Evaluation, Surveillance Training of Personnel − Strengthening Adverse Events Following Immunization (AEFI) systems, especially for older children − ‘Enhanced’ post-introduction evaluation in selected countries due to difficulty of complex surveillance on routine basis [high sensitization requirement or cultural misperception] − Collecting data, keeping track, and incorporating info into information record systems • Implementation training (e. g. , HCW) 28

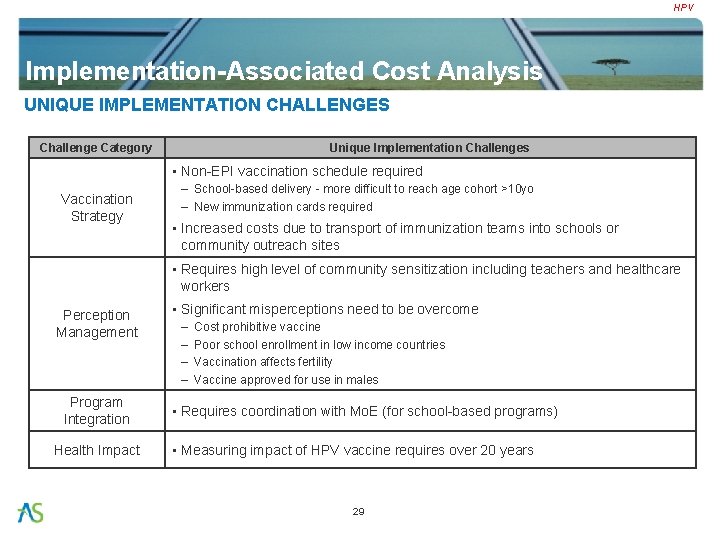
HPV Implementation-Associated Cost Analysis UNIQUE IMPLEMENTATION CHALLENGES Challenge Category Unique Implementation Challenges • Non-EPI vaccination schedule required Vaccination Strategy – School-based delivery - more difficult to reach age cohort >10 yo – New immunization cards required • Increased costs due to transport of immunization teams into schools or community outreach sites • Requires high level of community sensitization including teachers and healthcare workers Perception Management Program Integration Health Impact • Significant misperceptions need to be overcome – – Cost prohibitive vaccine Poor school enrollment in low income countries Vaccination affects fertility Vaccine approved for use in males • Requires coordination with Mo. E (for school-based programs) • Measuring impact of HPV vaccine requires over 20 years 29

HPV Implementation-Associated Cost Analysis POTENTIAL IMPLEMENTATION SYNERGIES Traditional Hep. B Hib YF Pneumo Rota Men. A HPV Cholera JE Rabies Rubella Typhoid √ Traditional = Routine EPI vaccines includes Baccillus Calmette-Guérin (BCG), Diphtheria-tetanus-pertussis (DTP) , measles containing vaccines (MCV), oral polio (OPV), Tetanus toxoid (TT) Vaccine-Specific Synergies • Potential to leverage school-based vaccination campaigns (cholera, typhoid) Other Potential Synergies • Potential to motivate the creation or expansion of adolescent health programs • Potential to integrate with gender-based initiatives 30 √

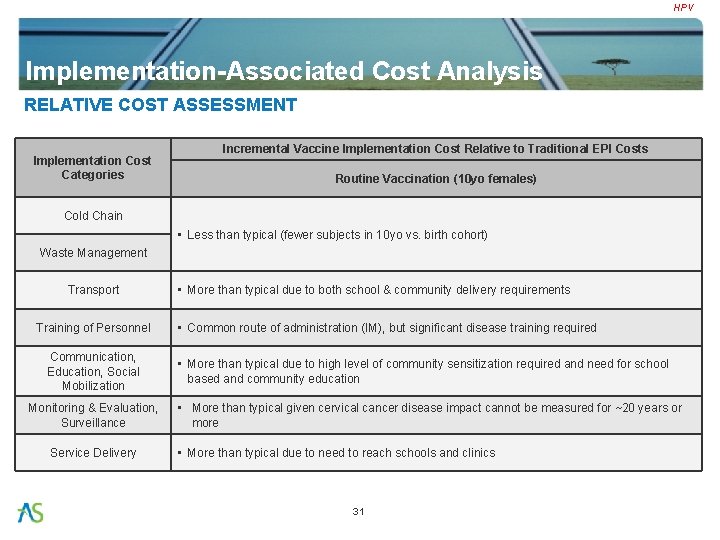
HPV Implementation-Associated Cost Analysis RELATIVE COST ASSESSMENT Implementation Cost Categories Incremental Vaccine Implementation Cost Relative to Traditional EPI Costs Routine Vaccination (10 yo females) Cold Chain • Less than typical (fewer subjects in 10 yo vs. birth cohort) Waste Management Transport Training of Personnel Communication, Education, Social Mobilization Monitoring & Evaluation, Surveillance Service Delivery • More than typical due to both school & community delivery requirements • Common route of administration (IM), but significant disease training required • More than typical due to high level of community sensitization required and need for school based and community education • More than typical given cervical cancer disease impact cannot be measured for ~20 years or more • More than typical due to need to reach schools and clinics 31

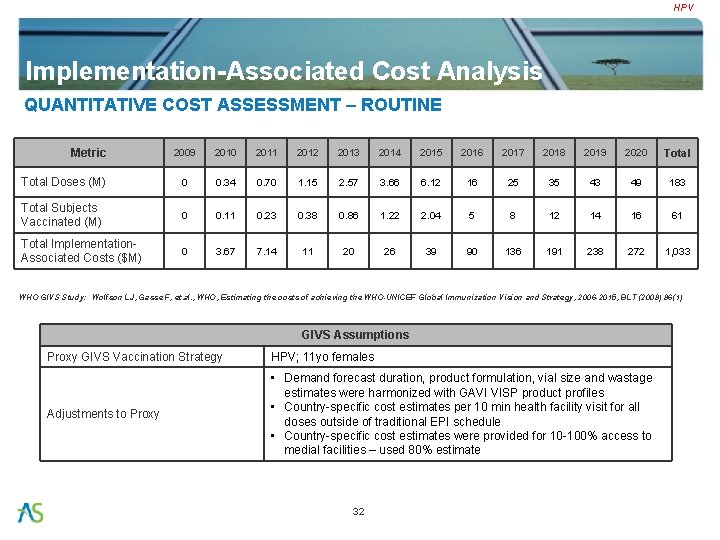
HPV Implementation-Associated Cost Analysis QUANTITATIVE COST ASSESSMENT – ROUTINE Metric 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Total Doses (M) 0 0. 34 0. 70 1. 15 2. 57 3. 66 6. 12 16 25 35 43 49 183 Total Subjects Vaccinated (M) 0 0. 11 0. 23 0. 38 0. 86 1. 22 2. 04 5 8 12 14 16 61 Total Implementation. Associated Costs ($M) 0 3. 67 7. 14 11 20 26 39 90 136 191 238 272 1, 033 WHO GIVS Study: Wolfson LJ, Gasse F, et. al. , WHO, Estimating the costs of achieving the WHO-UNICEF Global Immunization Vision and Strategy, 2006 -2015, BLT (2008) 86(1) GIVS Assumptions Proxy GIVS Vaccination Strategy HPV; 11 yo females Adjustments to Proxy • Demand forecast duration, product formulation, vial size and wastage estimates were harmonized with GAVI VISP product profiles • Country-specific cost estimates per 10 min health facility visit for all doses outside of traditional EPI schedule • Country-specific cost estimates were provided for 10 -100% access to medial facilities – used 80% estimate 32

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccine Need & Adoption Forecast • Vaccination Policy & Strategies • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 33

HPV Analysis Summary KEY METRIC SUMMARY Routine Vaccination Cost Component GAVI Vaccine Cost ($M) 1, 261 Country Vaccine Co-Pay Cost ($M) Total Vaccine Cost ($M) 31 $1, 293 Vaccination Strategy Deaths Averted <5 Deaths Averted Cases Averted Cost Savings GAVI Cost/ Death Averted GAVI Cost/ Case Averted GAVI Cost/ $ Saved Country Imp Costs Routine 707 n/a 870 $258 $1, 784 $1, 449 $4. 90 $1, 033 34

HPV CONTENTS • Disease Overview • Vaccine Landscape • Vaccine Need & Adoption Forecast • Vaccination Policy & Strategies • Vaccine Cost Analysis • Implementation-Associated Cost Analysis • Analysis Summary • Key Resources 35

HPV Key Resources EXPERT CONSULTATION • Product Development Program (PATH HPV Vaccine Team) – Vivien Tsu, Director – Steve Brooke, Commercialization Advisor, Commercialization & Corporate Partnerships – Debbie Atherly, Senior Health & Policy Officer – Carol Levin, Senior Health Economist – Nancy Engel • Independent Experts – Sue Goldie, Harvard School of Public Health • Suppliers – Merck: Margaret Mc. Glynn, President, Merck Vaccines; Elaine Esber, Executive Director, Merck Vaccines – GSK: John-Kenneth Billingsley, Director, Global Vaccine Policy & Public Partnerships; Eunice Miranda, Director, Global Commercial Affairs; Laurence de Moerlooze, Head of Project Management, HPV Vaccines; Kate Taylor, VP, Global Vaccine Policy & Public Health Partnerships; Kathleen Vandendael-Baudrihaye, Director, International Relations, Global Vaccine Policy & Public Health Partnerships • Others – Nuriye Ortayli, Advisor, Reproductive Health, UNFPA 36

HPV Appendix REFERENCES (I) 1. Plotkin, S, Orenstein, W, Offit, P. Vaccines, 5 th edition, Chapter 14, 2008 2. “HPV Disease and Epidemiology”, slide presentation by M. Stanley, Dept of Pathology, University of Cambridge 3. Smith et al, Int. J. Cancer 121: 621– 32, 2007 4. Cancer Survival in Developing Countries, by Sankaranarayanan et al, IARCP press, 1998 5. HPV vaccines: technical information for policy-makers and health professionals, WHO publication 2007 6. PATH HPV team 7. WHO/EPI Burden of Disease Projections (http: //www. who. int/healthinfo/statistics/bodprojections 2030/en/index. html) 8. Morbidity & Mortality data obtained through IARC, Globocan 2002 (http: //www. who. int/hpvcentre/statistics/dynamic/ico/Data. Query. Select. cfm) 9. Morbidity=number of new cervical cancer incident cases caused by HPV infection 10. Morbidity & Mortality rates based on the crude rates (per 100, 000 female population) from Globocan 2002 [Morbidity Rate = Crude Morbidity Rate] 37

HPV Appendix REFERENCES (II) 11. Mortality=number of death caused by HPV infection induced cervical cancer 12. [Mortality Rate = (Crude Mortality Rate / 100, 000) 1, 000] 13. Giuliano et al, J Infect Dis 188(10): 1508 -16, 2003 14. Goodman et al, Cancer Res 67(12): 5987 -96, 2007 15. Kwasniewska et al, Nutrition and cancer 28(3): 248 -51, 1997 16. PATH, Outlook vol. 23(1), June 2007 17. Some cross protection to HPV-45 & 31 using 16 & 18 shown (PATH, Outlook vol. 23(1), June 2007 ) 18. Gardasil Package Insert (http: //www. merck. com/product/usa/pi_circulars/g/gardasil_pi. pdf) 19. http: //www. dhs. ca. gov/director/owh_main/pubs_events/news_articles/YM/6. 2006 cervi calvaccineyouth. pdf 20. www. medicalnewstoday. com/articles/77039. php 38

HPV Appendix REFERENCES (III) 21. Both Merck and GSK conducting studies to determine safety and immunogenicity of a booster dose (Vaccine 25(26): 4931 -9, 2007 & www. clinicaltrials. gov/ct 2/show/ NCT 00546078? term=nct 0054 6078&rank=1, respectively); as well as safety and immunogenicity in HIV infected children or female adults (NCT 00339040 & NCT 00586339, respectively) 22. Cervarix Package Insert & personal communication with GSK (http: //emc. medicines. org. uk/emc/assets/c/html/displaydoc. asp? documentid=20207) 23. http: //www. in-pharmatechnologist. com/news/ng. asp? n=80037 -glaxosmithkline-merckcervarix-gardasil-cervical-cancer 24. Gao et al, "HEV and HPV Vaccine Development", 8 th DCVMN Annual Meeting Rio de Janiero, Brazil, 2007 25. http: //www. ystxm. com/en 26. http: //www. who. int/healthinfo/statistics/bodprojections 2030/en/index. html 39

HPV Appendix REFERENCES (IV) 27. H. Saxenian & R. Hecht, Stop Cervical Cancer: Accelerating Global Access to HPV Vaccines, Dec 2006, London (background paper from IAVI) 28. HPV Vaccine Adoption in Developing Countries: Cost and Financing Issues, Dec 2007, IAVI 40
 Vaccine investment strategy
Vaccine investment strategy Vaccine investment strategy
Vaccine investment strategy Gavi anesi
Gavi anesi Gavi nu
Gavi nu Gavi equipes atuais
Gavi equipes atuais Nmf immunisation
Nmf immunisation Gender schema theory
Gender schema theory Duke human vaccine institute
Duke human vaccine institute Fixed investment and inventory investment
Fixed investment and inventory investment Strategic human resource
Strategic human resource Investment banking strategy
Investment banking strategy Ibd investment strategy
Ibd investment strategy Land based investment strategy
Land based investment strategy Human resources return on investment
Human resources return on investment Investment orientation
Investment orientation Investment perspective of hrm
Investment perspective of hrm Human body systems final exam
Human body systems final exam Recruitment and selection question paper
Recruitment and selection question paper Financial investment analysis
Financial investment analysis Factors that complicate capital investment analysis
Factors that complicate capital investment analysis Financial investment analysis
Financial investment analysis Factors that complicate capital investment analysis
Factors that complicate capital investment analysis Investment analysis and portfolio management notes
Investment analysis and portfolio management notes Capital investment analysis is the process
Capital investment analysis is the process Factors that complicate capital investment analysis
Factors that complicate capital investment analysis Scope of investment analysis and portfolio management
Scope of investment analysis and portfolio management Investment analysis & portfolio management
Investment analysis & portfolio management Investment analysis and portfolio management course
Investment analysis and portfolio management course Definition of vaccine
Definition of vaccine Edible vaccines pros and cons
Edible vaccines pros and cons Global vaccine safety blueprint
Global vaccine safety blueprint Rabies incubation period dogs
Rabies incubation period dogs Bactivor
Bactivor Hep b series for adults
Hep b series for adults Zostavax reconstitution
Zostavax reconstitution Grits georgia immunization
Grits georgia immunization Hpv vaccine schedule adults
Hpv vaccine schedule adults Dr sears vaccine schedule
Dr sears vaccine schedule Next generation vaccine
Next generation vaccine Mcv4 vaccine schedule
Mcv4 vaccine schedule Vcxin
Vcxin































































