Rutherfords Model Ernest Rutherford 1871 1937 Review of

















- Slides: 25

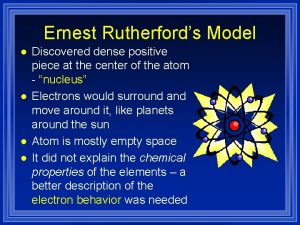
Rutherford’s Model Ernest Rutherford 1871 -1937

Review of Millikan’s Oil Drop Experiment § http: //highered. mcgrawhill. com/olcweb/cgi/pluginpop. cgi? it=swf: : 100% 25: : 100%25: : /sites/dl/free/0072512644/117354/ 02_Millikan_Oil_Drop. swf: : Milikan%20 Oil%20 D rop

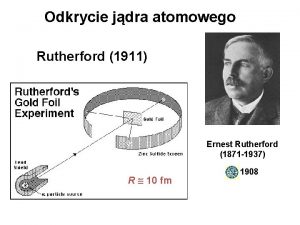
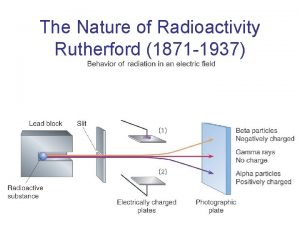
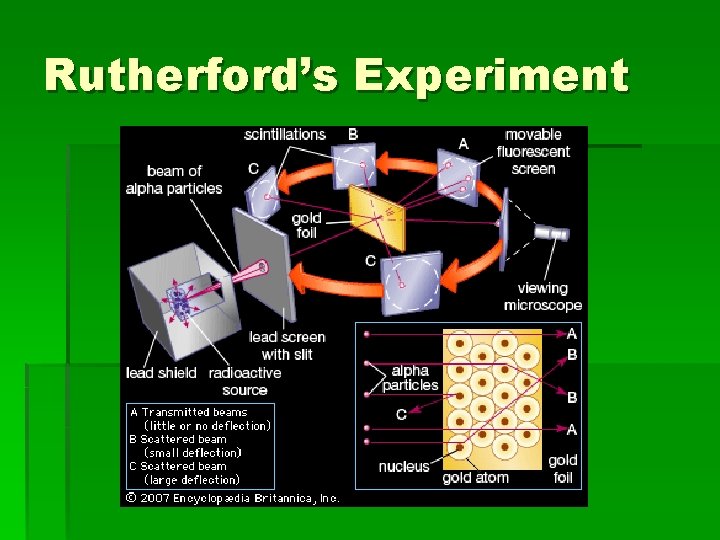
The Experiment § A beam of alpha particles fired at a sheet of very thin (approximately 400 atoms thick) gold foil.

Observation of the Experiment: § http: //www. mhhe. com/physsci/chemistry/ animations/chang_2 e/rutherfords_experi ment. swf

What should have happened: § If Thomson's Plum Pudding Model was correct, the positive charge of the atom is spread out and its effect would be very weak § should result in very small (if any!) scattering angles for the alpha particles, since the alpha particles would just about sail straight through.

What happened: § Most went straight through or deflected at small angles. § In rare cases particles were reflected straight back. Rutherford stated, “It was as if you fired a shell at tissue paper and it came back and hit you. ”

Rutherford’s Experiment

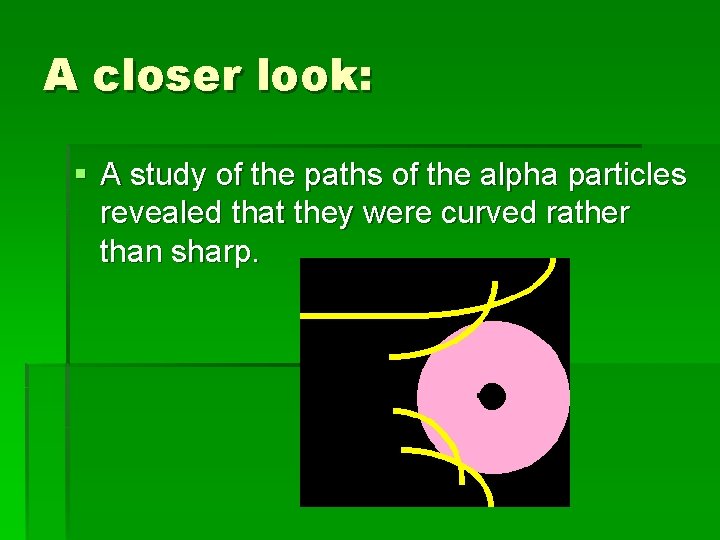
A closer look: § A study of the paths of the alpha particles revealed that they were curved rather than sharp.

Not a collision: § Rutherford deduced that the paths were most like objects being repelled by an electric force, not like those after a collision.

Positively charged part? ? ? § • This lead him to believe that there was a large positive charge repelling the positively charged alpha particles

Discovery of the Nucleus § He said that it must be in the center of the atom and he called it the nucleus.

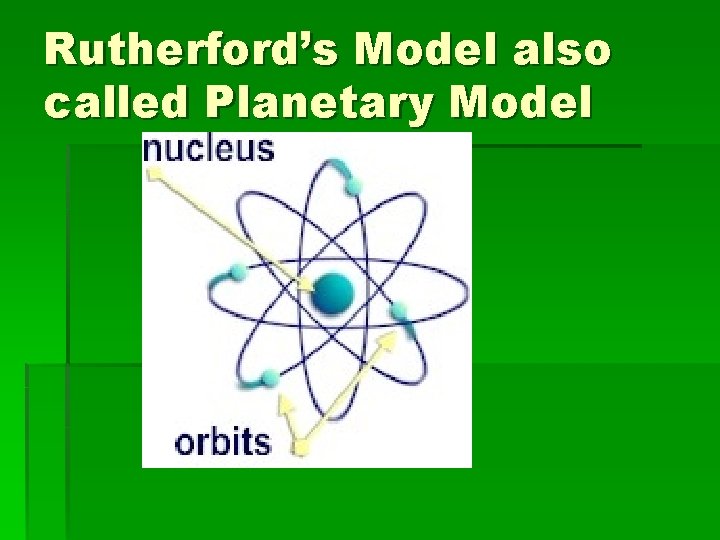
Rutherford’s Model also called Planetary Model

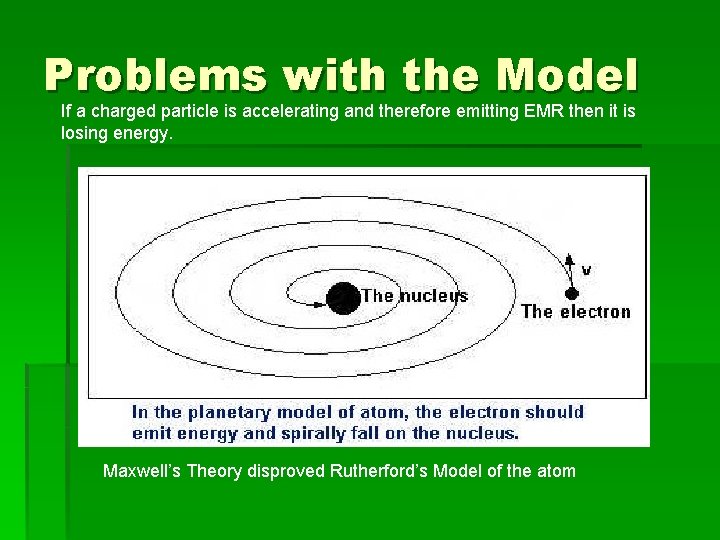
Problems with the Model If a charged particle is accelerating and therefore emitting EMR then it is losing energy. Maxwell’s Theory disproved Rutherford’s Model of the atom

Check up § Read p. 766 – 770 § Do Check and reflect p. 770 #1, 2, 4, 5

Bohr’s Model § Rutherford’s model was the only option until Bohr made some additional observations. § He decided that Planck’s idea of quantized energy would help explain the model of the atom Niels Bohr 1885 - 1962

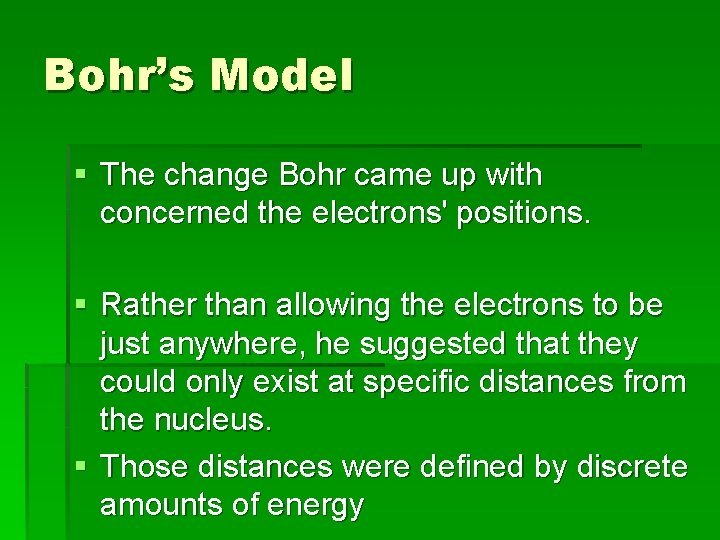
Bohr’s Model § The change Bohr came up with concerned the electrons' positions. § Rather than allowing the electrons to be just anywhere, he suggested that they could only exist at specific distances from the nucleus. § Those distances were defined by discrete amounts of energy

Remember EMR § As electrons spiral in they should go faster and release their energy in ever increasing amounts so we should observe increasingly higher frequencies of EMR being given off by atoms all the time § Instead, experiments showed that atoms only ever emit energy at certain frequencies

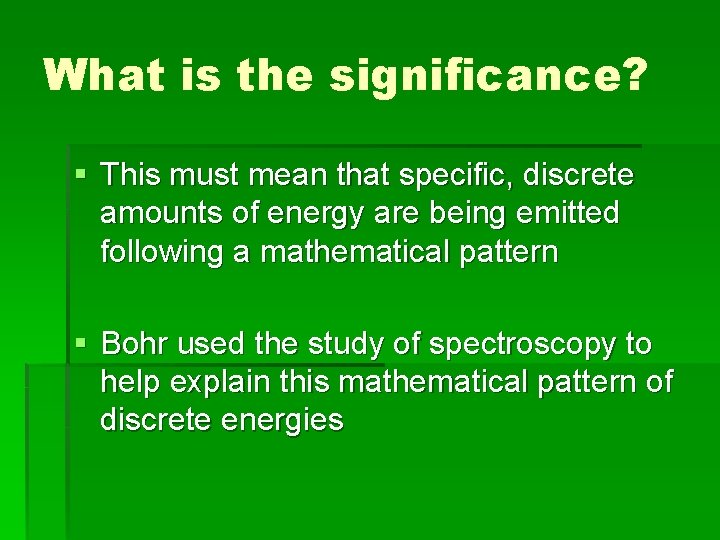
What is the significance? § This must mean that specific, discrete amounts of energy are being emitted following a mathematical pattern § Bohr used the study of spectroscopy to help explain this mathematical pattern of discrete energies

Spectroscopy involves looking at light from various sources through a diffraction grating and analyzing the colors that are seen.

Spectrum § Definition: a spectrum is the spread of values from a minimum to a maximum § The light spectrum is the wavelength or frequency values of visible EMR (700 nm to 400 nm) emitted by excited substances.

Continuous spectrum § is one in which EMR is given off when objects are heated to a high temperature § This is a full spectrum given off when a solid body, such as the element in a stove or incandescent light bulb, is excited.

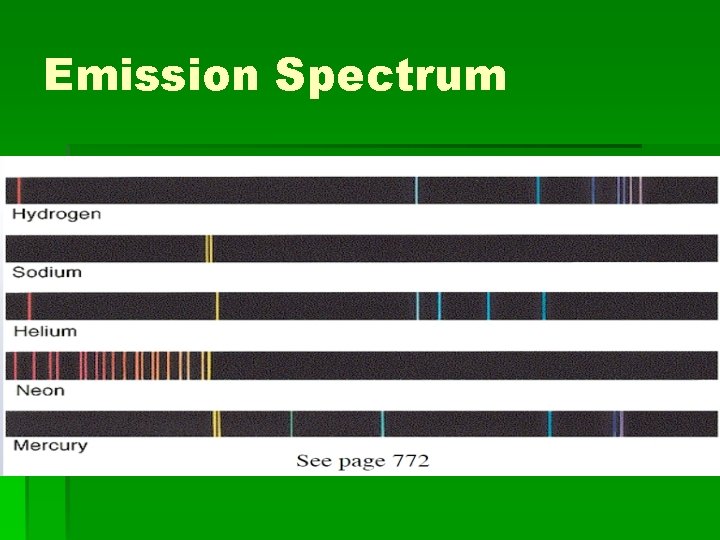
Emission spectrum of gases § As gases are excited, they give off only very discrete energies. This is why elements have certain colors when flame tested. § Each element will emit a certain set of wavelengths when it is heated.

Emission Spectrum

Absorption Spectrum § When light is passed through a cool gas only certain wavelengths are absorbed. § They are the same wavelengths that are emitted when the substance is excited.

 1937-1871
1937-1871 Atomic theory timeline
Atomic theory timeline Modelo atomico modelo actual
Modelo atomico modelo actual Rutherford streuversuch modell
Rutherford streuversuch modell Ernest rutherford steckbrief
Ernest rutherford steckbrief Características del modelo de thomson
Características del modelo de thomson Ernest rutherford
Ernest rutherford What did ernest rutherford discover
What did ernest rutherford discover Ernest rutherford objevy
Ernest rutherford objevy Ernest rutherford objevy
Ernest rutherford objevy Mapa conceptual de los modelos planetarios
Mapa conceptual de los modelos planetarios Rutherford gold foil
Rutherford gold foil Ernest rutherford atom modeli
Ernest rutherford atom modeli 1937 computer
1937 computer Contoh striving for superiority
Contoh striving for superiority Gerindo berdiri di jakarta 24 mei 1937 akibat dari
Gerindo berdiri di jakarta 24 mei 1937 akibat dari 1937
1937 U n i a c stands for
U n i a c stands for Ship with butterfly sails (1937) meaning
Ship with butterfly sails (1937) meaning July 2 1937 amelia earhart
July 2 1937 amelia earhart Aspirationòthe
Aspirationòthe Pablo picasso modernismen
Pablo picasso modernismen Teori atom
Teori atom Unification of germany class 10 mind map
Unification of germany class 10 mind map Charles babbage (1792-1871)
Charles babbage (1792-1871) Pengertian seni menurut eb taylor
Pengertian seni menurut eb taylor