Ernest Rutherford 1871 1937 Rutherford PAPER Learned physics


- Slides: 17

Ernest Rutherford (1871 -1937) Rutherford PAPER • Learned physics in J. J. Thomson’ lab. • Noticed that ‘alpha’ particles were sometime deflected by something in the air. • Gold-foil experiment Animation by Raymond Chang – All rights reserved.

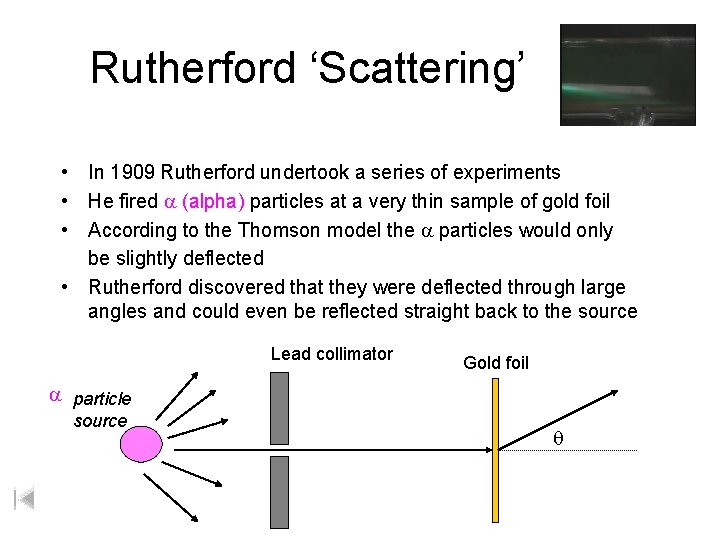
Rutherford ‘Scattering’ • In 1909 Rutherford undertook a series of experiments • He fired a (alpha) particles at a very thin sample of gold foil • According to the Thomson model the a particles would only be slightly deflected • Rutherford discovered that they were deflected through large angles and could even be reflected straight back to the source Lead collimator Gold foil a particle source q

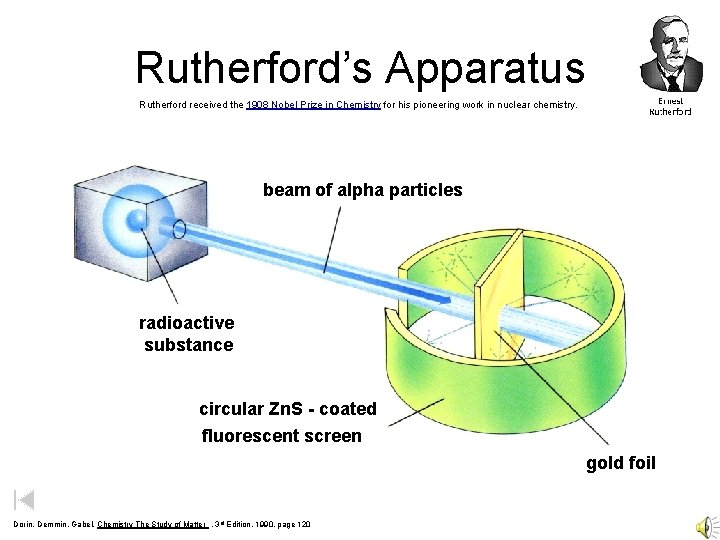
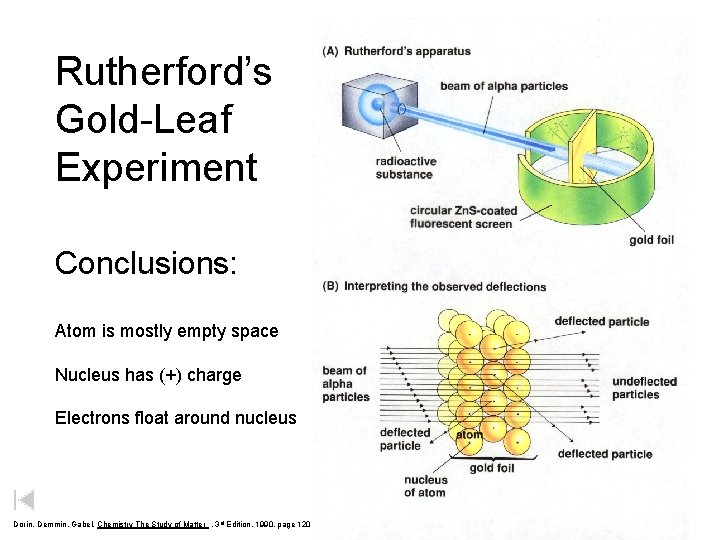
Rutherford’s Apparatus Rutherford received the 1908 Nobel Prize in Chemistry for his pioneering work in nuclear chemistry. beam of alpha particles radioactive substance circular Zn. S - coated fluorescent screen gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120

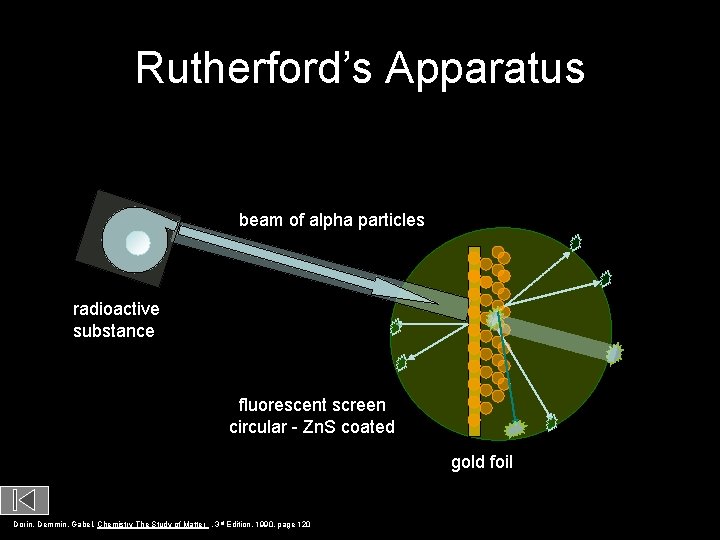
Rutherford’s Apparatus beam of alpha particles radioactive substance fluorescent screen circular - Zn. S coated gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120

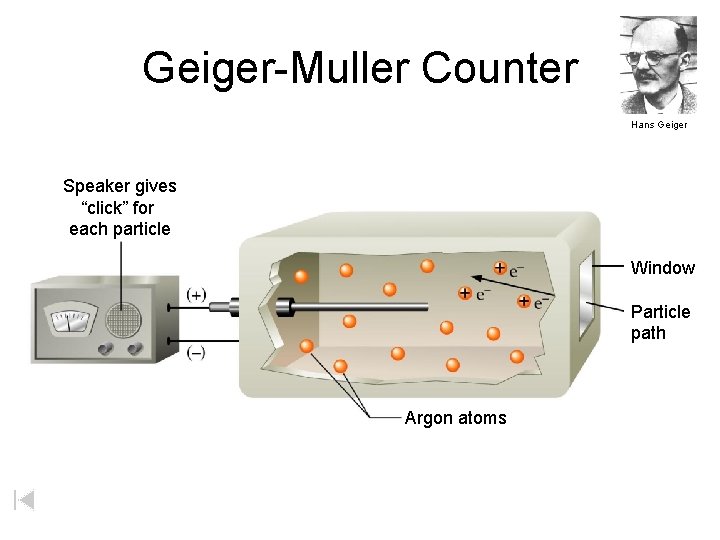
Geiger-Muller Counter Hans Geiger Speaker gives “click” for each particle Window Particle path Argon atoms

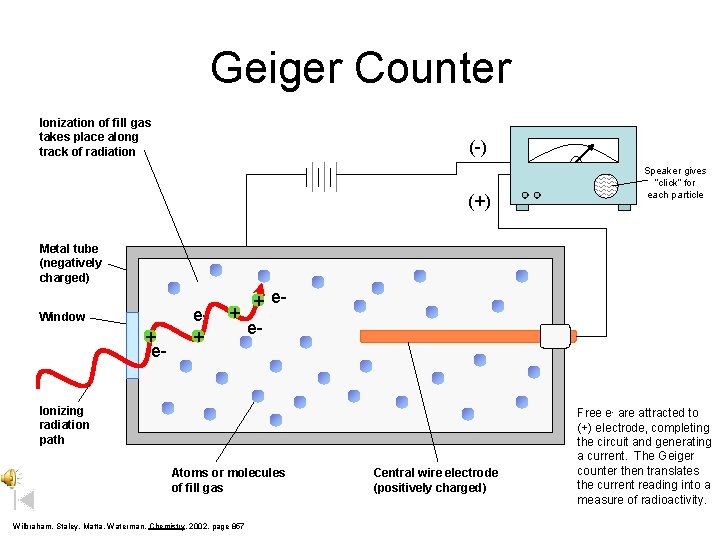
Geiger Counter Ionization of fill gas takes place along track of radiation (-) (+) Speaker gives “click” for each particle Metal tube (negatively charged) Window + e- e+ + + ee- Ionizing radiation path Atoms or molecules of fill gas Wilbraham, Staley, Matta, Waterman, Chemistry, 2002, page 857 Central wire electrode (positively charged) Free e- are attracted to (+) electrode, completing the circuit and generating a current. The Geiger counter then translates the current reading into a measure of radioactivity.

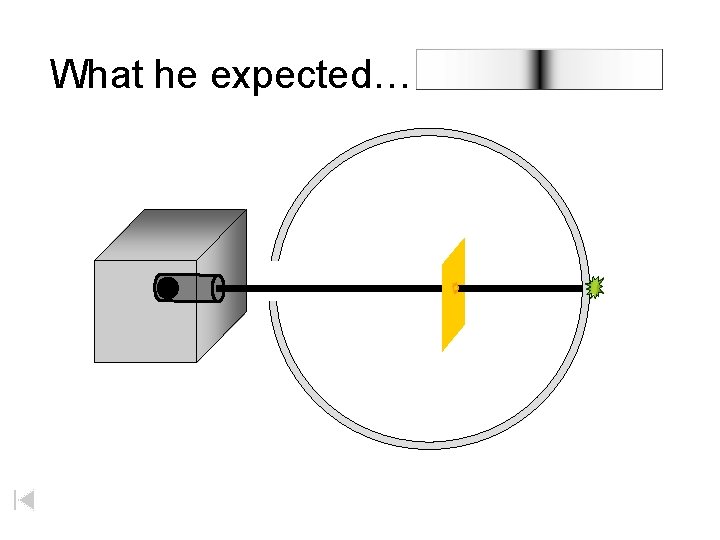
What he expected…

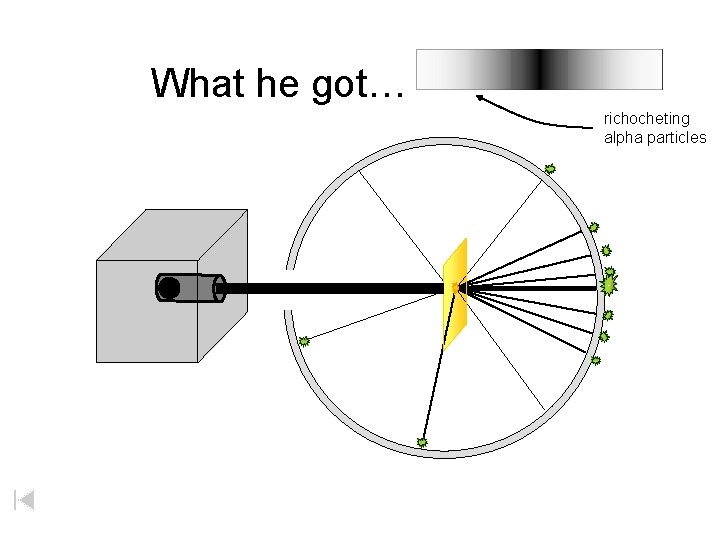
What he got… richocheting alpha particles

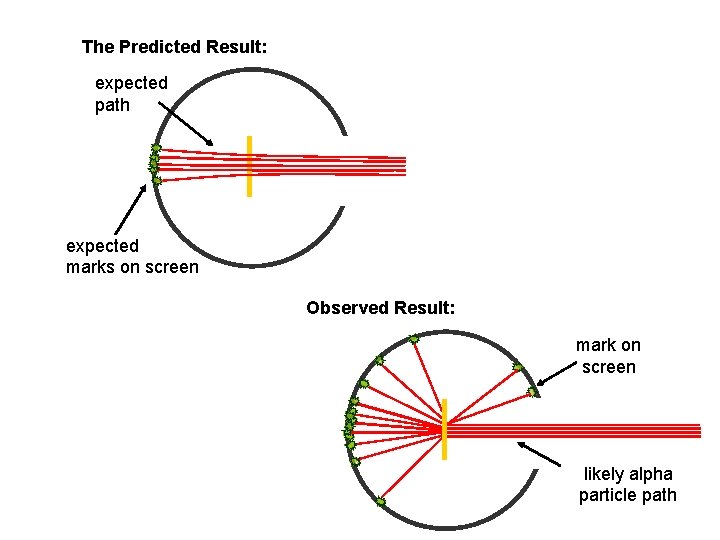
The Predicted Result: expected path expected marks on screen Observed Result: mark on screen likely alpha particle path

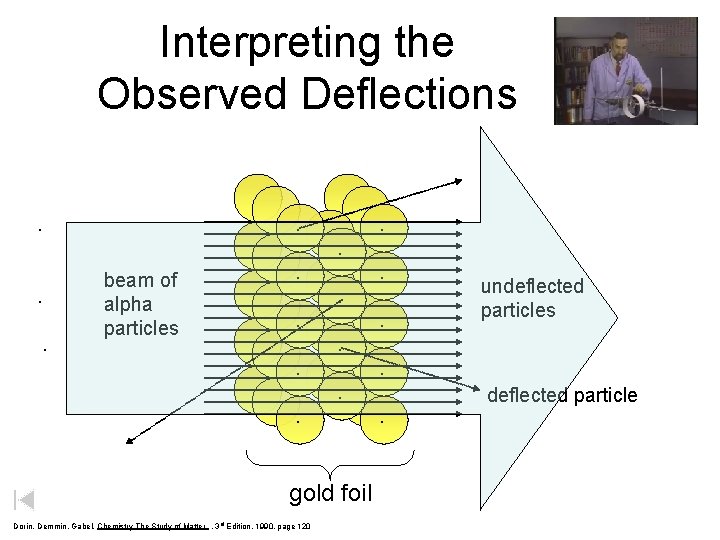
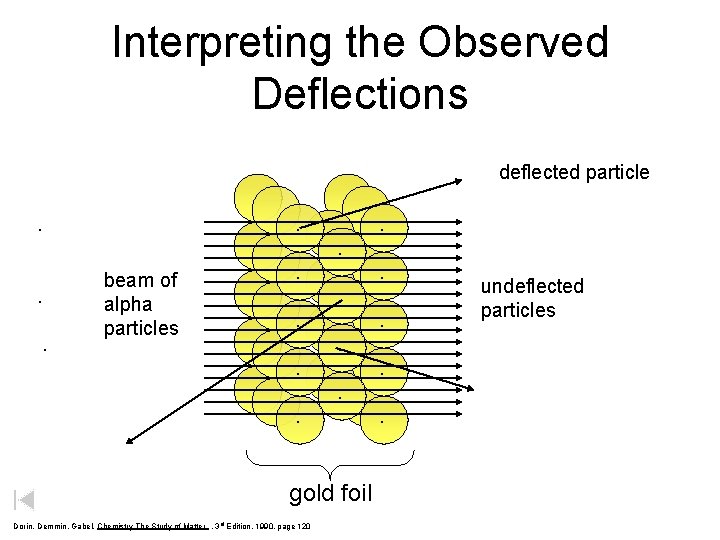
Interpreting the Observed Deflections. . beam of alpha particles . . . . gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120 . . . undeflected particles . . deflected particle

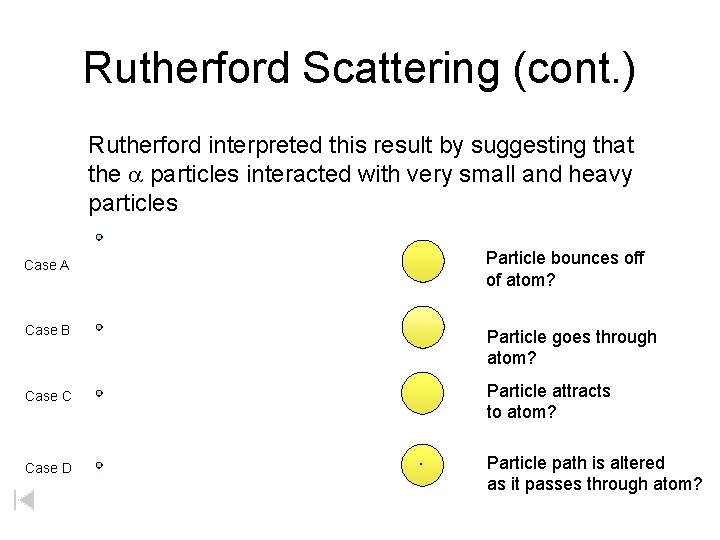
Rutherford Scattering (cont. ) Rutherford interpreted this result by suggesting that the a particles interacted with very small and heavy particles Particle bounces off of atom? Case A Case B Particle goes through atom? Particle attracts to atom? Case C Case D . Particle path is altered as it passes through atom?

Table: hypothetical description of alpha particles (based on properties of alpha radiation) observation hypothesis alpha rays don’t diffract . . . alpha radiation is a stream of particles alpha rays deflect towards a negatively charged plate and away from a positively charged plate . . . alpha particles have a positive charge alpha rays are deflected only slightly by an electric field; a cathode ray passing through the same field is deflected strongly . . . alpha particles either have much lower charge or much greater mass than electrons Copyright © 1997 -2005 by Fred Senese

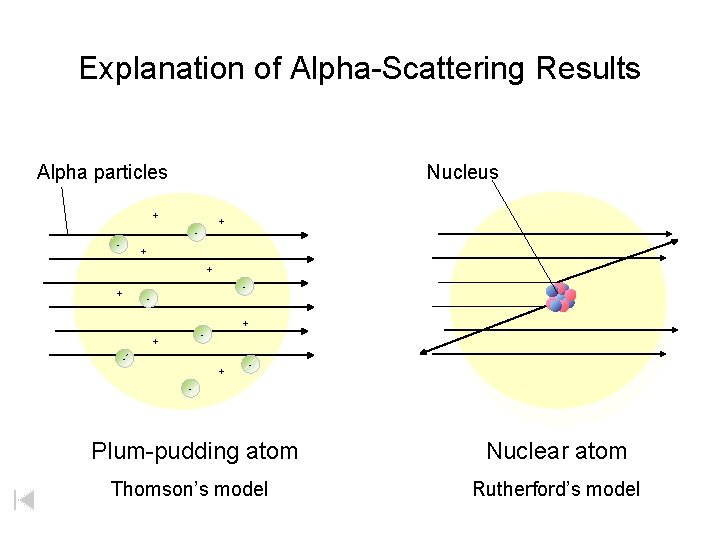
Explanation of Alpha-Scattering Results Alpha particles Nucleus + + - - + + - + - - Plum-pudding atom Nuclear atom Thomson’s model Rutherford’s model

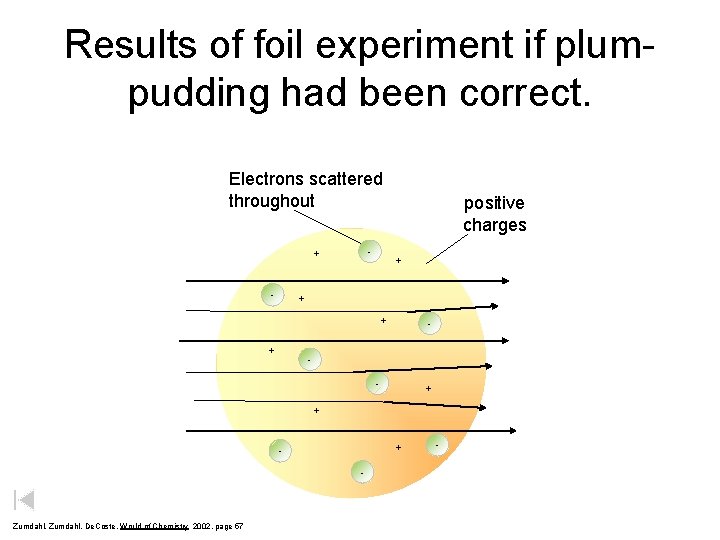
Results of foil experiment if plumpudding had been correct. Electrons scattered throughout - + - positive charges + + - - + + + - Zumdahl, De. Coste, World of Chemistry 2002, page 57 -

Interpreting the Observed Deflections deflected particle . . beam of alpha particles . . . . gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120 . . . undeflected particles

Rutherford’s Gold-Leaf Experiment Conclusions: Atom is mostly empty space Nucleus has (+) charge Electrons float around nucleus Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 120

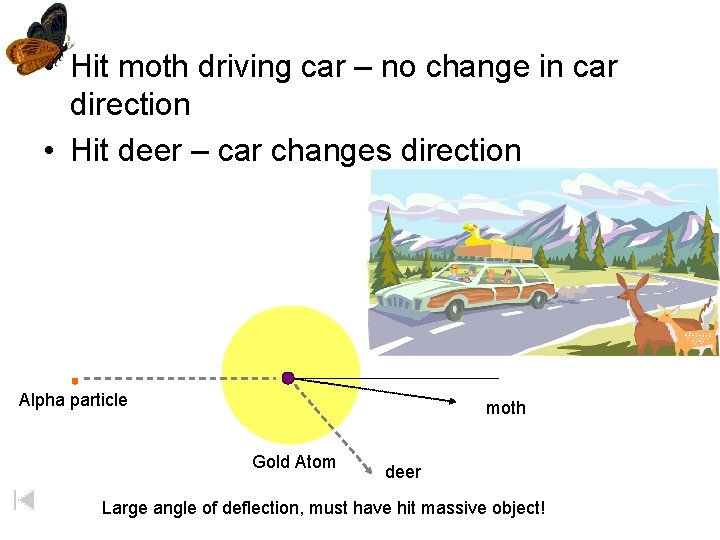
• Hit moth driving car – no change in car direction • Hit deer – car changes direction Alpha particle moth Gold Atom deer Large angle of deflection, must have hit massive object!