RETREATMENT OF HEPATITIS C VIRUS INFECTION IN PATIENTS






- Slides: 22

RETREATMENT OF HEPATITIS C VIRUS INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASVIR David Wyles, 1 Ola Weiland, 2 Betty Yao, 3 Robert Reindollar, 4 Frank Weilert, 5 Jean-Francois Dufour, 6 Stuart Gordon, 7 Fred Poordad, 8 Albrecht Stoehr, 9 Ashley St. John Mark Brown, 10 Stefan Mauss, 11 Suvajit Samanta, 3 Tami Pilot-Matias, 3 Lino Rodrigues Jr. , 3 Roger Trinh 3 1 Denver Health Medical Center, Denver, Colorado, USA; 2 Karolinska University Hospital Huddinge, Karolinska Institutet, Stockholm, Sweden; 3 Abb. Vie Inc. , North Chicago, Illinois, USA; 4 Piedmont Health. Care, Statesville, North Carolina, USA; 5 Waikato Hospital, Hamilton, New Zealand; 6 Inselspital, Bern, Switzerland; 7 Henry Ford Hospital, Detroit, Michigan, USA; 8 Texas Liver Institute, UT Health, San Antonio, Texas, USA; 9 IFI Studien und Projekte Gmbh, Hamburg, Germany; 10 Imperial College Healthcare NHS Trust, London, United Kingdom; 11 Center for HIV and Hepatogastroenterology, Düsseldorf, Germany Presented at the EASL International Liver Congress Paris, France 12 April 2018

Disclosures D Wyles: Research support from Abb. Vie, Gilead, and Merck; Consultant for Abb. Vie, Gilead, and Merck. O Weiland: Speaker/Consultant for Abb. Vie, Bristol-Myers Squibb, Gilead, Janssen, and MSD/Merck. R Reindollar: Research support from Abb. Vie, Bristol-Myers Squibb, Cepheid, Gilead, Intercept, and Janssen; Consultant/Speaker for Abb. Vie, Bristol-Myers Squibb, Gilead, and Janssen. F Weilert: Investigator in Abb. Vie supported study. J-F Dufour: Advisory committees for Abb. Vie, Bayer, BMS, Genfit, Gilead Science, Intercept, Merck, and Novartis; Unrestricted research grant from Bayer. S Gordon: Consultant for Abb. Vie, Bristol-Myers Squibb, CVS Caremark, Gilead, and Merck; Grant Support from Abb. Vie, Bristol-Myers Squibb, Gilead, Intercept, and Merck. F Poordad: Grant/research support from Abb. Vie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol. Myers Squibb, Genentech, Gilead Sciences, Glaxo. Smith. Kline, Globe. Immune, Idenix Pharmaceuticals, Idera Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Medarex, Medtronic, Merck, Novartis, Santaris Pharmaceuticals, Scynexis Pharmaceuticals, Vertex Pharmaceuticals, and Zymo. Genetics; Speaker for Gilead, Kadmon, Merck, Onyx/Bayer, Genentech, GSK, Salix, and Vertex; Consultant/advisor for Abb. Vie, Achillion Pharmaceuticals, Anadys Pharmaceuticals, Biolex Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo. Smith. Kline, Globe. Immune, Idenix, Merck, Novartis, Tibotec/Janssen, Theravance, and Vertex. A Stoehr: Speakers bureau for Abb. Vie, Gilead, Janssen, and MSD; Ad boards for Abb. Vie and Vii. V. A Brown: Advisor/speaker/recipient of research grants for Abbvie, Bristol-Meyers Squibb, Janssen, Gilead, and MSD S Mauss: Speakers bureau for Abb. Vie, Gilead, Falk, Janssen, and MSD; Advisory boards for Abb. Vie and MSD B Yao, S Samanta, T Pilot-Matias, L Rodrigues Jr, and R Trinh: employees of Abb. Vie and may hold stock or stock options. The design, study conduct, analysis, and financial support of the study (NCT 02939989) were provided by Abb. Vie participated in the interpretation of data, review, and approval of the content. All authors had access to all relevant data and participated in writing, review, and approval of this presentation. Medical writing was provided by Zoë Hunter, Ph. D of Abb. Vie.

Please note that, in Germany, Glecaprevir/Pibrentasvir (G/P) is currently not approved for retreatment of patients who failed previous direct-acting antiviral therapy

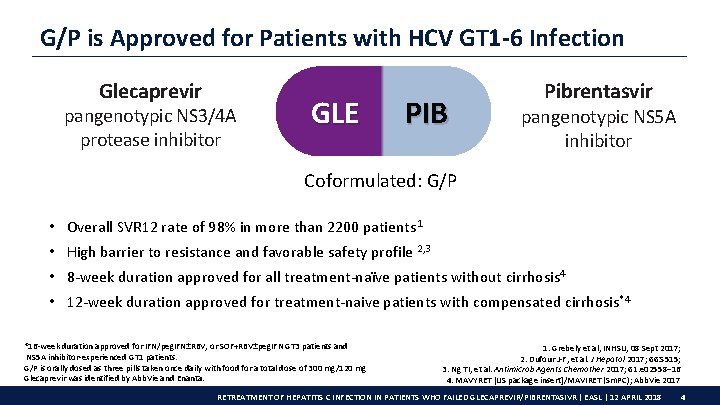
G/P is Approved for Patients with HCV GT 1 -6 Infection Glecaprevir pangenotypic NS 3/4 A protease inhibitor GLE PIB Pibrentasvir pangenotypic NS 5 A inhibitor Coformulated: G/P • Overall SVR 12 rate of 98% in more than 2200 patients 1 • High barrier to resistance and favorable safety profile 2, 3 • 8 -week duration approved for all treatment-naïve patients without cirrhosis 4 • 12 -week duration approved for treatment-naive patients with compensated cirrhosis*4 *16 -week duration approved for IFN/peg. IFN±RBV, or SOF+RBV±peg. IFN GT 3 patients and NS 5 A inhibitor-experienced GT 1 patients. G/P is orally dosed as three pills taken once daily with food for a total dose of 300 mg/120 mg Glecaprevir was identified by Abb. Vie and Enanta. 1. Grebely et al, INHSU, 08 Sept 2017; 2. Dufour J-F, et al. J Hepatol 2017; 66: S 515; 3. Ng TI, et al. Antimicrob Agents Chemother 2017; 61: e 02558– 16 4. MAVYRET [US package insert]/MAVIRET (Sm. PC); Abb. Vie 2017 RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 4

Background Combining drugs with different mechanisms of action can increase the probability of achieving a sustained virologic response (SVR) in HCV-infected patients who have previously failed DAA treatment, especially with NS 5 A-containing regimens 1 -5 MAGELLAN-3 is an ongoing study evaluating the safety and efficacy of G/P in combination with sofosbuvir (SOF) and ribavirin (RBV) as a retreatment regimen for patients from Abb. Vie’s registrational program who experienced virologic failure (VF) with G/P 1. Sulkowsi MS, Gardiner DF, et al. N. Engl. J. Med. 2014; 370: 211– 221 2. Zeuzem S, Jacobson IM, et al. N. Engl. J. Med. 2014; 370: 1604– 1614. 3. Hezode C, Fourati S, et al. J Hepatology. 2016; 64: S 400 4. Poordad F, Bennett M, et al. J Hepatology. 2016; 64(2): S 767 5. Gane EJ, Kowdley KV, et al. Gastroenterology. 2016; 151: 902– 909 RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 5

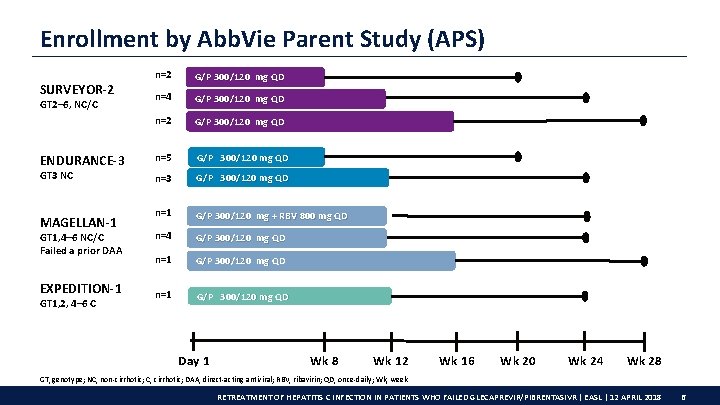
Enrollment by Abb. Vie Parent Study (APS) SURVEYOR-2 GT 2– 6, NC/C ENDURANCE-3 GT 3 NC MAGELLAN-1 GT 1, 4– 6 NC/C Failed a prior DAA EXPEDITION-1 GT 1, 2, 4– 6 C n=2 G/P 300/120 mg QD n=4 G/P 300/120 mg QD n=2 G/P 300/120 mg QD n=5 G/P 300/120 mg QD n=3 G/P 300/120 mg QD n=1 G/P 300/120 mg + RBV 800 mg QD n=4 G/P 300/120 mg QD n=1 G/P 300/120 mg QD Day 1 Wk 8 Wk 12 Wk 16 Wk 20 Wk 24 Wk 28 GT, genotype; NC, non-cirrhotic; C, cirrhotic; DAA, direct-acting antiviral; RBV, ribavirin; QD, once-daily; Wk, week RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 6

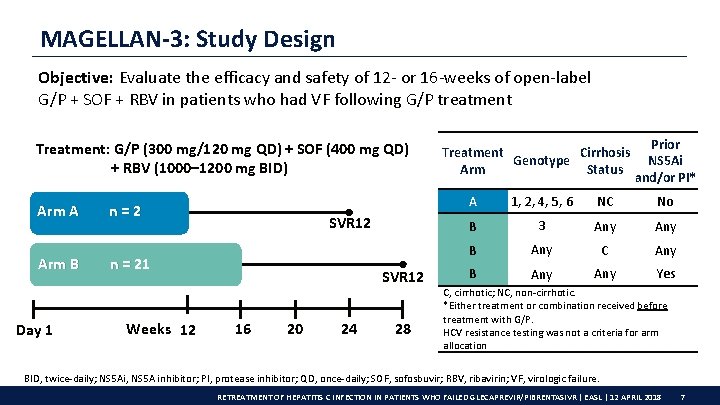
MAGELLAN-3: Study Design Objective: Evaluate the efficacy and safety of 12 - or 16 -weeks of open-label G/P + SOF + RBV in patients who had VF following G/P treatment Treatment: G/P (300 mg/120 mg QD) + SOF (400 mg QD) + RBV (1000– 1200 mg BID) Arm A Arm B Day 1 n=2 SVR 12 n = 21 Weeks 12 SVR 12 16 20 24 28 Treatment Cirrhosis Genotype Arm Status Prior NS 5 Ai and/or PI* A 1, 2, 4, 5, 6 NC No B 3 Any B Any C Any B Any Yes C, cirrhotic; NC, non-cirrhotic. *Either treatment or combination received before treatment with G/P. HCV resistance testing was not a criteria for arm allocation BID, twice-daily; NS 5 Ai, NS 5 A inhibitor; PI, protease inhibitor; QD, once-daily; SOF, sofosbuvir; RBV, ribavirin; VF, virologic failure. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 7

Key Patient Eligibility Criteria • Age ≥ 18 years • HCV GT 1– 6, with or without HIV-1 co-infection (ART-naïve or on stable ART) • Experienced VF during or after treatment with G/P in an Abbvie clinical study • G/P treatment was completed or discontinued ≥ 1 month prior to screening • Absence of co-infection with hepatitis B virus • Absence of decompensated cirrhosis (Child-Pugh B/C) ART, anti-retroviral therapy. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 8

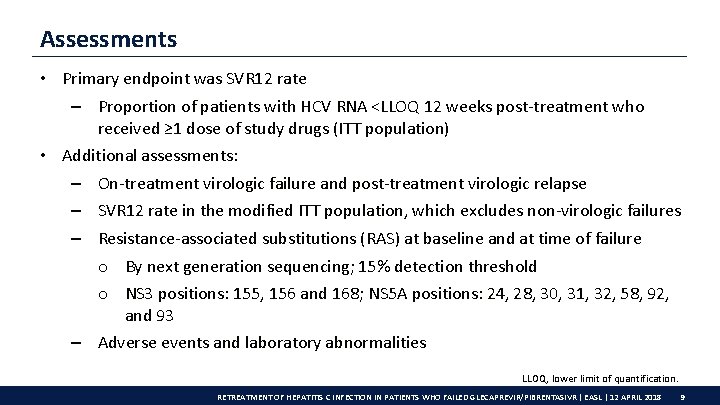
Assessments • Primary endpoint was SVR 12 rate – Proportion of patients with HCV RNA <LLOQ 12 weeks post-treatment who received ≥ 1 dose of study drugs (ITT population) • Additional assessments: – On-treatment virologic failure and post-treatment virologic relapse – SVR 12 rate in the modified ITT population, which excludes non-virologic failures – Resistance-associated substitutions (RAS) at baseline and at time of failure o By next generation sequencing; 15% detection threshold o NS 3 positions: 155, 156 and 168; NS 5 A positions: 24, 28, 30, 31, 32, 58, 92, and 93 – Adverse events and laboratory abnormalities LLOQ, lower limit of quantification. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 9

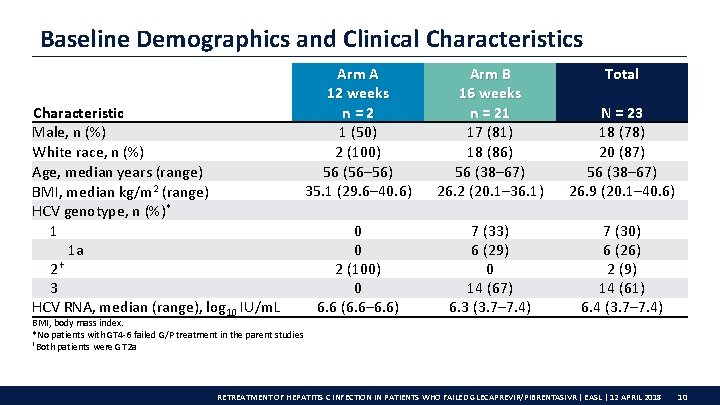
Baseline Demographics and Clinical Characteristics Characteristic Male, n (%) White race, n (%) Age, median years (range) BMI, median kg/m 2 (range) HCV genotype, n (%)* 1 1 a 2† 3 HCV RNA, median (range), log 10 IU/m. L BMI, body mass index. *No patients with GT 4 -6 failed G/P treatment in the parent studies †Both patients were GT 2 a Arm A 12 weeks n=2 1 (50) 2 (100) 56 (56– 56) 35. 1 (29. 6– 40. 6) Arm B 16 weeks n = 21 17 (81) 18 (86) 56 (38– 67) 26. 2 (20. 1– 36. 1) Total N = 23 18 (78) 20 (87) 56 (38– 67) 26. 9 (20. 1– 40. 6) 0 0 2 (100) 0 6. 6 (6. 6– 6. 6) 7 (33) 6 (29) 0 14 (67) 6. 3 (3. 7– 7. 4) 7 (30) 6 (26) 2 (9) 14 (61) 6. 4 (3. 7– 7. 4) RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 10

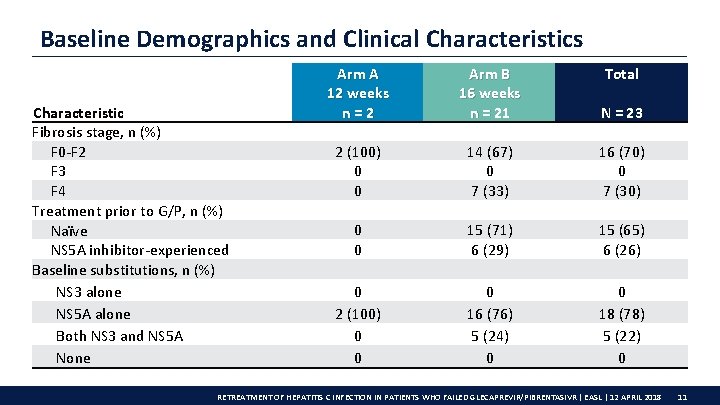
Baseline Demographics and Clinical Characteristics Characteristic Fibrosis stage, n (%) F 0 -F 2 F 3 F 4 Treatment prior to G/P, n (%) Naïve NS 5 A inhibitor-experienced Baseline substitutions, n (%) NS 3 alone NS 5 A alone Both NS 3 and NS 5 A None Arm A 12 weeks n=2 Arm B 16 weeks n = 21 Total N = 23 2 (100) 0 0 14 (67) 0 7 (33) 16 (70) 0 7 (30) 0 0 15 (71) 6 (29) 15 (65) 6 (26) 0 2 (100) 0 0 0 16 (76) 5 (24) 0 0 18 (78) 5 (22) 0 RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 11

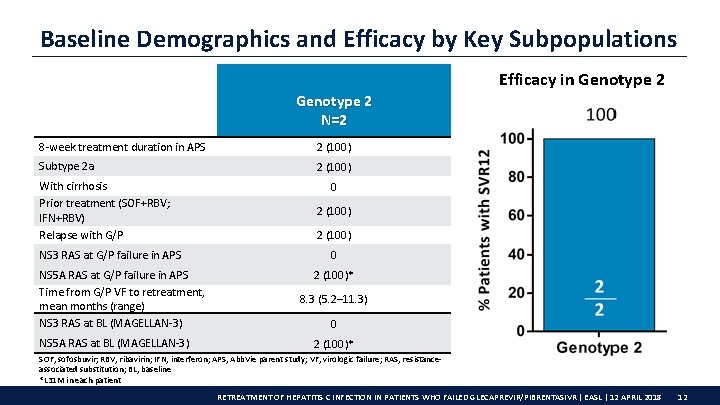
Baseline Demographics and Efficacy by Key Subpopulations Efficacy in Genotype 2 N=2 8 -week treatment duration in APS 2 (100) Subtype 2 a 2 (100) With cirrhosis Prior treatment (SOF+RBV; IFN+RBV) Relapse with G/P NS 3 RAS at G/P failure in APS NS 5 A RAS at G/P failure in APS Time from G/P VF to retreatment, mean months (range) NS 3 RAS at BL (MAGELLAN-3) NS 5 A RAS at BL (MAGELLAN-3) 0 2 (100)* 8. 3 (5. 2– 11. 3) 0 2 (100)* SOF, sofosbuvir; RBV, ribavirin; IFN, interferon; APS, Abb. Vie parent study; VF, virologic failure; RAS, resistanceassociated substitution; BL, baseline *L 31 M in each patient RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 12

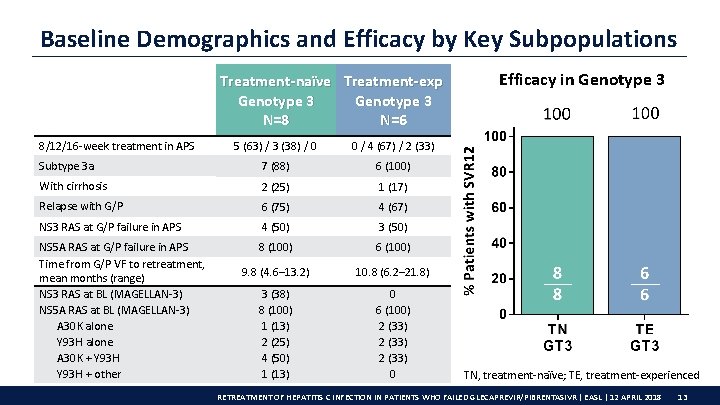
Baseline Demographics and Efficacy by Key Subpopulations Treatment-naïve Treatment-exp Genotype 3 N=8 N=6 8/12/16 -week treatment in APS 5 (63) / 3 (38) / 0 0 / 4 (67) / 2 (33) Subtype 3 a 7 (88) 6 (100) With cirrhosis 2 (25) 1 (17) Relapse with G/P 6 (75) 4 (67) NS 3 RAS at G/P failure in APS 4 (50) 3 (50) NS 5 A RAS at G/P failure in APS Time from G/P VF to retreatment, mean months (range) NS 3 RAS at BL (MAGELLAN-3) NS 5 A RAS at BL (MAGELLAN-3) A 30 K alone Y 93 H alone A 30 K + Y 93 H + other 8 (100) 6 (100) 9. 8 (4. 6– 13. 2) 10. 8 (6. 2– 21. 8) 3 (38) 8 (100) 1 (13) 2 (25) 4 (50) 1 (13) 0 6 (100) 2 (33) 0 Efficacy in Genotype 3 TN, treatment-naïve; TE, treatment-experienced RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 13

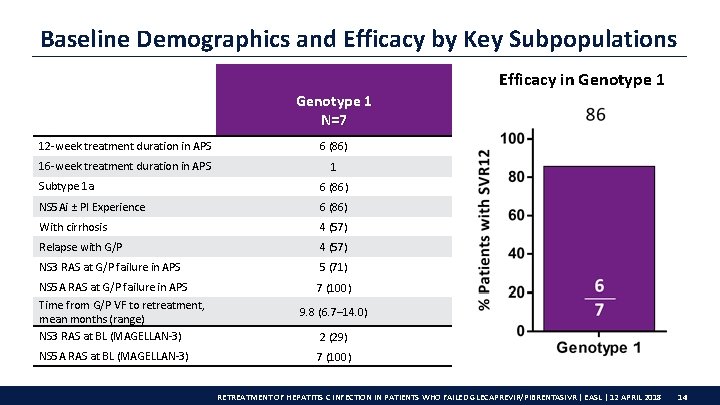
Baseline Demographics and Efficacy by Key Subpopulations Efficacy in Genotype 1 N=7 12 -week treatment duration in APS 6 (86) 16 -week treatment duration in APS 1 Subtype 1 a 6 (86) NS 5 Ai ± PI Experience 6 (86) With cirrhosis 4 (57) Relapse with G/P 4 (57) NS 3 RAS at G/P failure in APS 5 (71) NS 5 A RAS at G/P failure in APS Time from G/P VF to retreatment, mean months (range) NS 3 RAS at BL (MAGELLAN-3) 7 (100) NS 5 A RAS at BL (MAGELLAN-3) 9. 8 (6. 7– 14. 0) 2 (29) 7 (100) RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 14

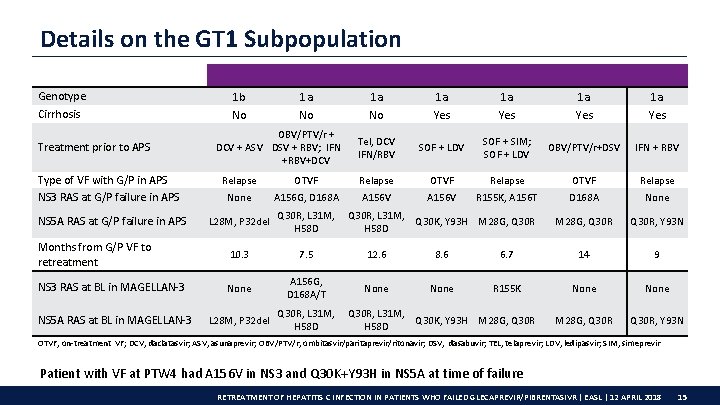
Details on the GT 1 Subpopulation Genotype Cirrhosis Treatment prior to APS Type of VF with G/P in APS NS 3 RAS at G/P failure in APS NS 5 A RAS at G/P failure in APS 1 b No 1 a No OBV/PTV/r + DCV + ASV DSV + RBV; IFN +RBV+DCV 1 a No 1 a Yes Tel, DCV IFN/RBV SOF + LDV SOF + SIM; SOF + LDV OBV/PTV/r+DSV IFN + RBV Relapse OTVF Relapse None A 156 G, D 168 A A 156 V R 155 K, A 156 T D 168 A None M 28 G, Q 30 R, Y 93 N L 28 M, P 32 del Q 30 R, L 31 M, Q 30 K, Y 93 H M 28 G, Q 30 R H 58 D Months from G/P VF to retreatment 10. 3 7. 5 12. 6 8. 6 6. 7 14 9 NS 3 RAS at BL in MAGELLAN-3 None A 156 G, D 168 A/T None R 155 K None M 28 G, Q 30 R, Y 93 N NS 5 A RAS at BL in MAGELLAN-3 L 28 M, P 32 del Q 30 R, L 31 M, Q 30 K, Y 93 H M 28 G, Q 30 R H 58 D OTVF, on-treatment VF; DCV, daclatasvir; ASV, asunaprevir; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; DSV, dasabuvir; TEL, telaprevir; LDV, ledipasvir; SIM, simeprevir Patient with VF at PTW 4 had A 156 V in NS 3 and Q 30 K+Y 93 H in NS 5 A at time of failure RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 15

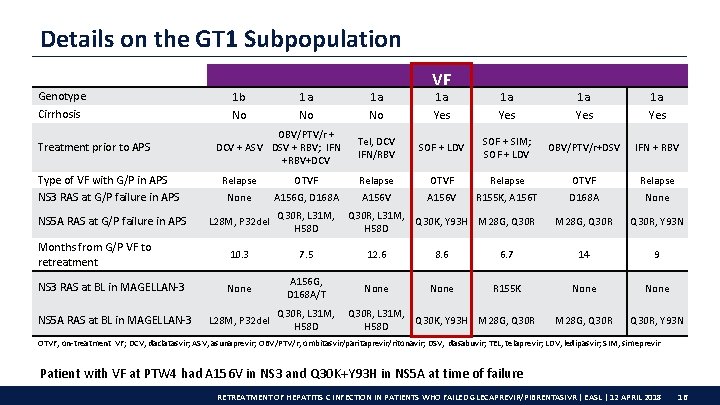
Details on the GT 1 Subpopulation Genotype Cirrhosis Treatment prior to APS Type of VF with G/P in APS NS 3 RAS at G/P failure in APS NS 5 A RAS at G/P failure in APS 1 b No 1 a No OBV/PTV/r + DCV + ASV DSV + RBV; IFN +RBV+DCV 1 a No VF 1 a Yes Tel, DCV IFN/RBV SOF + LDV SOF + SIM; SOF + LDV OBV/PTV/r+DSV IFN + RBV Relapse OTVF Relapse None A 156 G, D 168 A A 156 V R 155 K, A 156 T D 168 A None M 28 G, Q 30 R, Y 93 N L 28 M, P 32 del Q 30 R, L 31 M, Q 30 K, Y 93 H M 28 G, Q 30 R H 58 D Months from G/P VF to retreatment 10. 3 7. 5 12. 6 8. 6 6. 7 14 9 NS 3 RAS at BL in MAGELLAN-3 None A 156 G, D 168 A/T None R 155 K None M 28 G, Q 30 R, Y 93 N NS 5 A RAS at BL in MAGELLAN-3 L 28 M, P 32 del Q 30 R, L 31 M, Q 30 K, Y 93 H M 28 G, Q 30 R H 58 D OTVF, on-treatment VF; DCV, daclatasvir; ASV, asunaprevir; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; DSV, dasabuvir; TEL, telaprevir; LDV, ledipasvir; SIM, simeprevir Patient with VF at PTW 4 had A 156 V in NS 3 and Q 30 K+Y 93 H in NS 5 A at time of failure RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 16

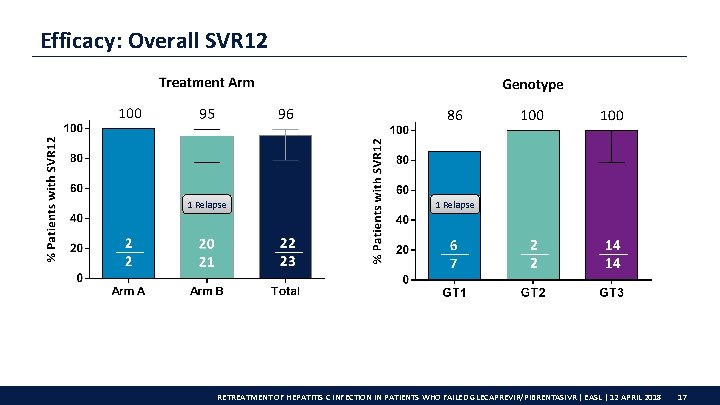
Efficacy: Overall SVR 12 1 Relapse RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 17

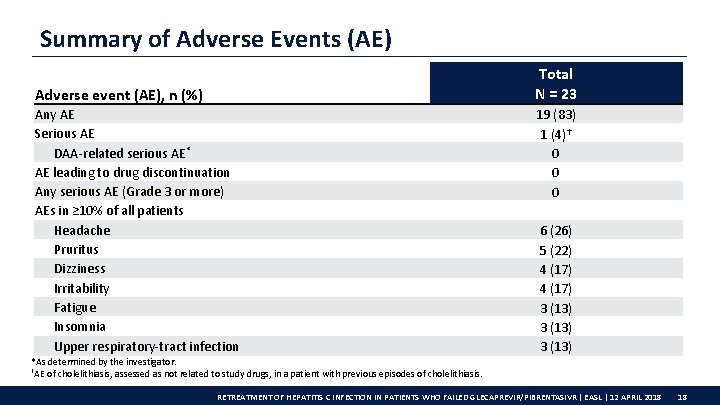
Summary of Adverse Events (AE) Total N = 23 Adverse event (AE), n (%) Any AE Serious AE DAA-related serious AE* AE leading to drug discontinuation Any serious AE (Grade 3 or more) AEs in ≥ 10% of all patients Headache Pruritus Dizziness Irritability Fatigue Insomnia Upper respiratory-tract infection 19 (83) 1 (4)† 0 0 0 6 (26) 5 (22) 4 (17) 3 (13) *As determined by the investigator. †AE of cholelithiasis, assessed as not related to study drugs, in a patient with previous episodes of cholelithiasis. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 18

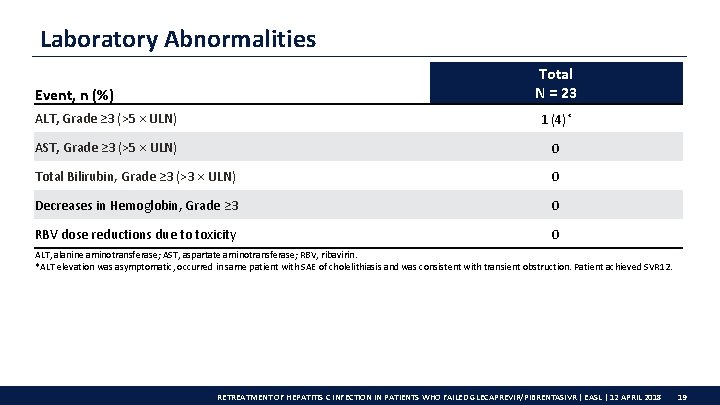
Laboratory Abnormalities Total N = 23 Event, n (%) ALT, Grade ≥ 3 (>5 × ULN) 1 (4)* AST, Grade ≥ 3 (>5 × ULN) 0 Total Bilirubin, Grade ≥ 3 (>3 ULN) 0 Decreases in Hemoglobin, Grade ≥ 3 0 RBV dose reductions due to toxicity 0 ALT, alanine aminotransferase; AST, aspartate aminotransferase; RBV, ribavirin. *ALT elevation was asymptomatic, occurred in same patient with SAE of cholelithiasis and was consistent with transient obstruction. Patient achieved SVR 12. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 19

Conclusions Overall rate of VF with G/P treatment is low; these results show that patients who fail G/P treatment can be successfully retreated • 96% SVR 12 rate in G/P VFs following retreatment with G/P + SOF + RBV • 100% (14/14) SVR 12 in GT 3 G/P + SOF + RBV was well tolerated: • No discontinuations • No DAA-related serious AEs • No RBV dose reductions due to toxicity This study is ongoing; patients who experienced VF from selected phase 3 b trials are eligible for participation RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 20

Acknowledgement The authors would like to express their gratitude to the patients who participated in this study and their families, as well as the investigators and study-site coordinators. RETREATMENT OF HEPATITIS C INFECTION IN PATIENTS WHO FAILED GLECAPREVIR/PIBRENTASIVR | EASL | 12 APRIL 2018 21

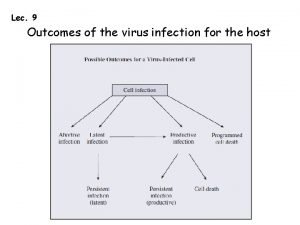
 Virus infection
Virus infection Pictures of body lice and scabies
Pictures of body lice and scabies Question one piece
Question one piece Colestasis gestacional
Colestasis gestacional Hepatitis b panel
Hepatitis b panel Causes of chronic hepatitis
Causes of chronic hepatitis Achrolic
Achrolic Klasifikasi hepatitis a
Klasifikasi hepatitis a Hepatitis lupica
Hepatitis lupica Hepatitis b
Hepatitis b Hepatomogaly
Hepatomogaly Dosis de lactulosa en encefalopatia hepatica
Dosis de lactulosa en encefalopatia hepatica Estigmas de hepatopatía crónica
Estigmas de hepatopatía crónica Std refers to *
Std refers to * Alcoholic hepatitis
Alcoholic hepatitis Hepatitis c symptoms in men
Hepatitis c symptoms in men Hbv
Hbv Hepatitis b vaccine schedule for adults
Hepatitis b vaccine schedule for adults Hepatitis e
Hepatitis e Hepatitis
Hepatitis Ano ang pagkakaiba ng hepatitis a at b
Ano ang pagkakaiba ng hepatitis a at b Patho
Patho Porta hepatis
Porta hepatis