Recent Trends in Drug and Device Regulation Panelists

































- Slides: 38

Recent Trends in Drug and Device Regulation

Panelists • Isabel Duffy Associate Vice President Merck & Co. , Inc. • Jeff Handwerker Partner Arnold & Porter LLP • Michael Labson Partner Covington & Burling LLP • Bryant Lim Chief Compliance Officer Incyte Corporation

Important Information The opinions and comments expressed during this panel are solely those of the participants and not necessarily those of any current or former employers.

Topics • A Look Back… – Developments in Guidance from the OIG • A Look Ahead… – Update on 21 st Century Cures Act – Debate between regulatory framework & First Amendment

Coupons/Co-Pay Cards • Coupons have come under increasing scrutiny from a kickback and litigation perspective • Characteristics of coupons/co-pay cards: – Reductions in patient out-of-pocket cost for a drug without regard to financial need – Often offered to help offset high co-payment amounts set by formulary committees – Can be made in the form of paper coupons, co-pay cards or debit cards – Typically structured as “for as low as”, percentage off, and fixed amount off out-of-pocket cost – Coupons often are multiple times use 5

Coupons/Co-Pay Cards • Private Payor Litigation: – Parallel suits by private health benefit plan, on behalf of putative classes of payers – Allegations: • Coupon programs caused beneficiaries and physicians to choose higher priced drugs • Coupons should be treated as discounts in reported list price – Legal Framework • RICO and conspiracy to commit RICO • Tortious interference with a contract • All cases now dismissed 6

OIG Guidance Re: Coupons • Manufacturer Copayment Coupon Programs – On September 18, 2014, the OIG published a report titled, “Manufacturer Safeguards May Not Prevent Copayment Coupon Use for Part D Drugs, ” which discusses the results of a study of 30 manufacturers of the top 100 Part D brand -name drugs with coupons and with the highest Medicare expenditures. – On the same day, the OIG published a special advisory bulletin on Pharmaceutical Manufacturer Copayment Coupon Programs. 7

Summary of OIG Guidance Re: Coupons • Manufacturers’ current safeguards may not prevent all coupons from being used for drugs paid for Part D. CMS cited a third-party report suggesting a 6 -7% error rate. – Part D plans and other entities cannot identify coupons from the NCPDP claim forms submitted by pharmacies. • Manufacturers offering coupons may be subject to sanctions if they fail to take appropriate steps to ensure that such coupons are not used by federal program beneficiaries. – Failure to take such steps may be evidence of intent to induce the purchase of drugs paid for by federal healthcare programs. • Manufacturers may engage industry stakeholders, including CMS, in an effort to identify a solution to ensure that coupons are not used for drugs paid for by Part D. • Manufacturers and pharmacies ultimately bear the responsibility to operate these programs in compliance with federal law.

Patient Assistance Programs (PAPs) • There is no regulatory safe harbor for PAPs. • 2005 Special Advisory Bulletin: OIG recognizes the value of PAPs providing “important safety net assistance” to patients in need, but cautions that cost-sharing subsidies to federal program beneficiaries provided by manufacturer PAPs pose heightened risk of fraud and abuse under the anti -kickback statute – Why? - value provided to patients to induce a purchase of a reimbursable product • Two types are permitted: (1) Independent Foundations; (2) Manufacturer. Sponsored PAPs 9

Independent Foundation PAPs • Late 2013: published reports that manufacturers using charitable donations for commercial purposes – Seeking Alpha – NY Times – Senator Grassley • OIG investigation ensued, with meetings with various large foundations (e. g. CDF, PAN, etc. ) – required to re-certify by end of 2015 • May 2015: Qui Tam against Biogen Idec – alleges $349 M in donations in one year to CDF for MS funds 10

Independent Foundation PAPs • Donations from a manufacturer to an independent, bona fide charity PAP that provides cost-sharing to federal program beneficiaries should not raise anti-kickback concerns, provided: – Neither the manufacturer, nor an affiliate, exerts any direct or indirect influence or control over the charity or the subsidy program, – The charity awards assistance in a truly independent manner that severs any link between the manufacturer’s funding and the beneficiary, – The charity awards assistance without regard to the manufacturer’s interests and without regard to the beneficiary’s choice of product or healthcare provider, and – The manufacturer does not solicit or receive data from the charity that would facilitate correlating the amount or frequency of donations with the number of subsidized prescriptions for its products 11

Independent Charity PAPs • In May 2014, OIG issued new guidance document: reemphasized the need for independence between donors and charities • Narrowly tailored disease funds that cover few products, or that apply differing eligibility criteria, benefit designs, or offlabel policies for certain funds but not others, will be scrutinized closely to determine if the fund structures are for the benefit of a particular donor or otherwise serve as a conduit between a manufacturer and its patients – Specialty therapeutic funds are worth watching – Key is for fund to be defined consistent with “widely recognized clinical standards” and “in a manner that covers a broad spectrum of available drugs” 12

Manufacturer-Sponsored PAPs • Only free product; not co-pay subsidies • Should either exclude federal program beneficiaries or be structured such that free product is provided “outside of the federal program benefit” – Patient must not be able to seek reimbursement from the federal healthcare program and Medicare Part D beneficiaries (if applicable) must not count the cost of the free product towards True Out of Pocket Costs (Tr. OOP) (for Medicare, one option is to enter a data sharing agreement with CMS). – Any federal program beneficiaries who get assistance would need to stay in the PAP for at least the program year, but potentially for life of therapy. – If Medicaid or Medicare subsequently covers the cost of the product, there is a risk that the PAP could be viewed as a means to steer patients to the drug. The above features (e. g. a data sharing agreement, operating outside of the benefit, etc. ) mitigate this risk. • OIG Advisory Opinion 14 -05 – Reduced price program for cash paying patients for product post-LOE 13

Topics • A Look Back… – Developments in Guidance from the OIG • A Look Ahead… – Update on 21 st Century Cures Act – Debate between regulatory framework & First Amendment

21 st Century Cures Bill (H. R. 6) • Tit. I – Discovery • Tit. II – Development • Tit. III – Delivery

21 st Century Cures Bill (H. R. 6) – Key Drug Development Provisions • Structured risk-benefit framework (sec. 2001) • Use of patient experience data (sec. 2001) • Qualification of drug development tools (sec. 2021) • Accelerated approval development plans (sec. 2022) • Precision medicine guidance (sec. 2041) • Application of Bayesian statistics and adaptive trial designs (sec. 2061) • Utilizing clinical experience evidence (sec. 2062) • Streamlined data review (sec. 2063)

21 st Century Cures Bill (H. R. 6) – Key Drug Development Provisions • Sec. 2121 – Approval of antibiotics for limited patient populations • Sec. 2153 – Reauthorization of rare pediatric disease priority review voucher • Sec. 2151 – Orphan drug exclusivity extension (6 mos. ) • Sec. 2181 – Enhancing combination product review

21 st Century Cures Bill (H. R. 6) – Expanded Access • Sec. 2082 – Establishes new FDCA Sec. 561 A – Manufacturer must make publicly available its policy on responding to requests for expanded access, including: • • Contact information Procedures Criteria for granting requests Timing

21 st Century Cures Bill (H. R. 6) – Manufacturer Communications • Sec. 2101 – Healthcare Economic Information – Expanded permitted audience – Information must “relate” to an approved indication (vs. “directly relate”) – “Health care economic information” includes the “clinical data, inputs, clinical or other assumptions, methods, results, and other components underlying or comprising the analysis” • May be based on the separate or aggregated clinical consequences of the represented health outcomes • May be comparative to the use of another drug

21 st Century Cures Bill (H. R. 6) – Manufacturer Communications • Sec. 2102 – Scientific and Medical Developments: – “Not later than 18 months after the date of enactment of this Act, the Secretary of Health and Human Services shall issue draft guidance on facilitating the responsible dissemination of truthful and non-misleading scientific and medical information not included in the approved labeling of drugs and devices. ”

Topics • A Look Back… – Developments in Guidance from the OIG • A Look Ahead… – Update on 21 st Century Cures Act – Debate between regulatory framework & First Amendment

A Look Ahead… The Free Speech Debate Continues • The debate between: – Regulatory definition of speech in the form of ‘labeling’ and ‘advertising; ’ and – Commercial speech under the First Amendment

Regulation of Drug Promotion Under the Food, Drug, and Cosmetic Act (FDCA) FDCA sec. 502 – Misbranding • Labeling is “false or misleading in any particular” • Labeling lacks “adequate directions for use” • Advertisement lacks “true statement” in “brief summary” re side effects, contraindications, and effectiveness 23

The Off-Label Dilemma • Off-label use may be important for patient care – Doctors may prescribe approved drugs for unapproved uses – Approved labeling may lag medical science – Off label use may be accepted medical practice, supported by literature, and reimbursable BUT • Companies may not promote for these uses 24

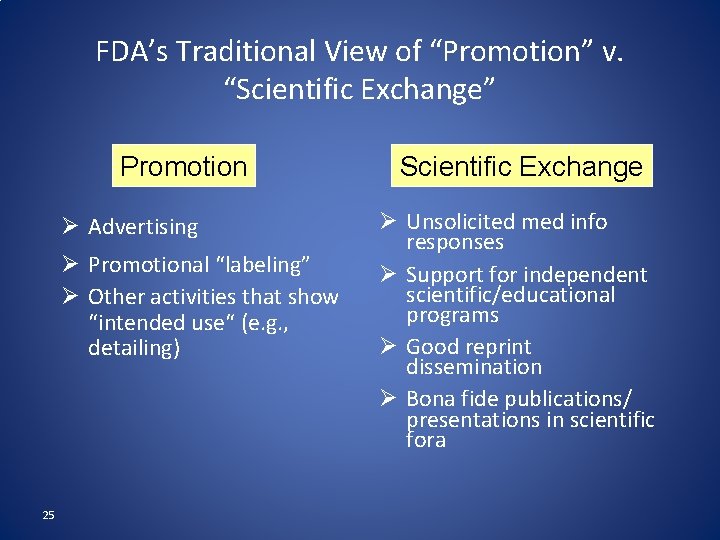
FDA’s Traditional View of “Promotion” v. “Scientific Exchange” Promotion Ø Advertising Ø Promotional “labeling” Ø Other activities that show “intended use“ (e. g. , detailing) 25 Scientific Exchange Ø Unsolicited med info responses Ø Support for independent scientific/educational programs Ø Good reprint dissemination Ø Bona fide publications/ presentations in scientific fora

First Amendment Tests • Strict Scrutiny: Speech restriction must further a compelling interest and be narrowly tailored to serve that interest – Political and non-commercial speech, such as scientific exchange – Potentially mixed commercial and non-commercial speech • Intermediate Scrutiny/Central Hudson: Regulation of truthful and non-misleading speech must directly and materially further an important government interest in a manner no more restrictive of speech than necessary to achieve the government interest – Commercial speech • Rational Basis: Regulation reasonably related to any hypothetical government interest – Applies to Government regulation of conduct 26

Generally Accepted First Amendment Principles • Corporations have full First Amendment rights – Citizens United v. Fed. Elec. Comm’n, 130 S. Ct. 876 (2010) • Free marketplace of ideas – best response to speech is counter-speech, not censorship • Paternalism is not a valid government interest – Thompson v. Western States Med. Ctr. , 535 U. S. 357 (2002) (rejecting “the notion that the Government has an interest in preventing the dissemination of truthful commercial information in order to prevent members of the public from making bad decisions with the information. ” ) – Va. State Bd. of Pharmacy, 425 U. S. at 773 (1976) (Laws based on the fear that truthful information will have an “effect upon. . . its recipients” have long been inherently suspect) • Hostility to a message is not a valid government interest – Snyder v. Phelps, 131 S. Ct. 1207, 1219 (2011) (picketing at military funerals) 27

Viewpoint discrimination is presumptively invalid • Speech restrictions that discriminate based on the identity of the speaker or the content of the message are presumptively invalid – Simon & Schuster, Inc. v. Members of the N. Y. State Crime Victims Bd. , 502 U. S. 105, 116 (1991) (Son of Sam law). – R. A. V. v. City of St. Paul, 505 U. S. 377, 392 (1992) (the government may not “license one side of a debate to fight freestyle, while requiring the other to follow Marquis of Queensberry rules”) – Rosenberger v. Rector & Visitors of the Univ. of Va. , 515 U. S. 819, 829 (1995) • The Government can no more burden speech based on the content of its message than censor it – Minneapolis Star & Tribune Co. v. Minnesota Commissioner of Revenue, 460 U. S. 575, 592 -93 (1983) (ink tax on some newspapers but not others violated First Amendment) 28

IMS v. Sorrell • IMS v. Sorrell invalidated under the First Amendment a Vermont law that banned pharmaceutical sales representatives from using prescriberidentifying data when marketing drugs to physicians The Court held that the law discriminated against content and speaker, triggering heightened scrutiny. Kennedy “Speech in aid of pharmaceutical marketing is a form of expression protected by the First Amendment. ” The law failed Central Hudson because it did not directly advance the government’s interest and was more extensive than necessary. The Court opens a Pandora's Box of First Amendment challenges. Breyer 29

Importance of Sorrell • Supreme Court decision in Sorrell confirms pharmaceutical companies’ First Amendment right to promote their products – “Lawmakers may no more silence the unwanted speech by burdening its utterance than by censoring its content. ” – “If pharmaceutical marketing affects treatment decisions, it does so because doctors find it persuasive. Absent circumstances far from those presented here, the fear that speech might persuade provides no lawful basis for quieting it. ” – “But the fear that people would make bad decisions if given truthful information cannot justify content-based burdens on speech. ” – “That the State finds expression too persuasive does not permit it to quiet the speech or to burden its messengers. ” 30

United States v. Caronia: The Alleged Misbranding of Xyrem • Xyrem is manufactured by Orphan Medical, now Jazz Pharmaceuticals • Approved by the FDA to treat narcolepsy and excessive day time sleepiness • Not approved for patients under 16 or the elderly 31

The Trial “Caronia is promoting, he’s marketing a dangerous drug for use not approved by the FDA. ” “He knew the rules: you can’t promote and market Xyrem for uses that have not been approved by the FDA. He admits it. ” “Caronia was promoting, trying to get physicians prescribe Xyrem. ” “A misbranded drug may be shown by a promotion of the drug for an intended use different from that approved by the FDA. ” Oops! My bad. But promotion is speech protected by the First Amendment. The government is trying to make protected speech the basis for criminal liability. 32

The Verdict • The jury found Caronia guilty of conspiracy to introduce into interstate commerce a misbranded drug • The court sentenced Caronia to one year probation, 100 hours of community service, and a $25 special assessment 33

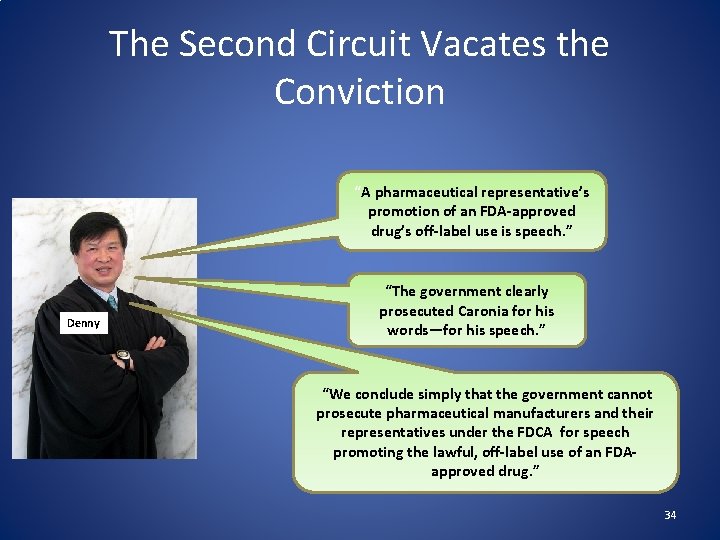
The Second Circuit Vacates the Conviction “A pharmaceutical representative’s promotion of an FDA-approved drug’s off-label use is speech. ” Denny “The government clearly prosecuted Caronia for his words—for his speech. ” “We conclude simply that the government cannot prosecute pharmaceutical manufacturers and their representatives under the FDCA for speech promoting the lawful, off-label use of an FDAapproved drug. ” 34

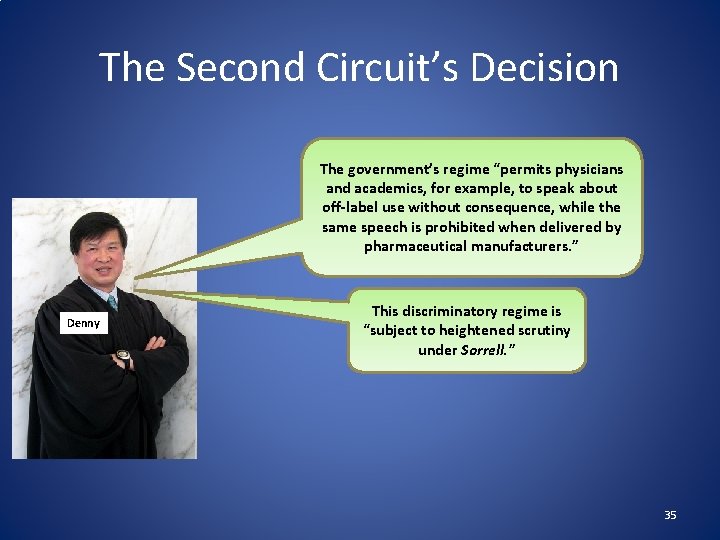
The Second Circuit’s Decision The government’s regime “permits physicians and academics, for example, to speak about off-label use without consequence, while the same speech is prohibited when delivered by pharmaceutical manufacturers. ” Denny This discriminatory regime is “subject to heightened scrutiny under Sorrell. ” 35

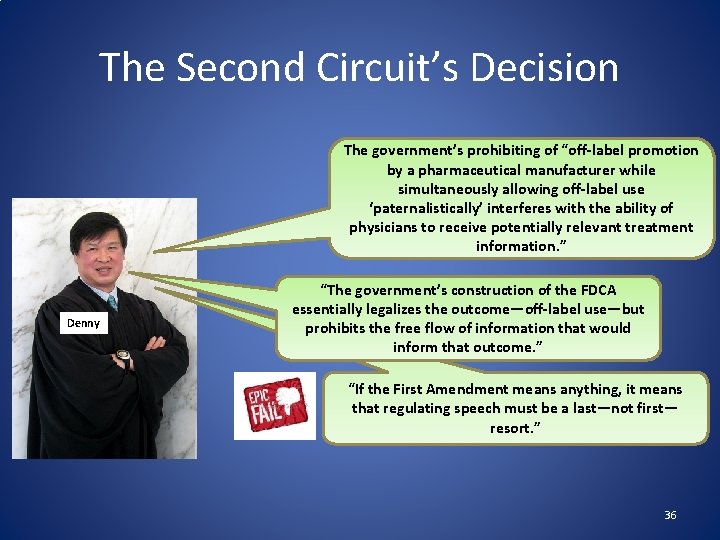
The Second Circuit’s Decision The government’s prohibiting of “off-label promotion by a pharmaceutical manufacturer while simultaneously allowing off-label use ‘paternalistically’ interferes with the ability of physicians to receive potentially relevant treatment information. ” Denny “The government’s construction of the FDCA essentially legalizes the outcome—off-label use—but prohibits the free flow of information that would inform that outcome. ” “If the First Amendment means anything, it means that regulating speech must be a last—not first— resort. ” 36

Subsequent Cases • Most recent: Amarin (SDNY) – Manufactures Vascepa, an omega-3 fatty acid • Approved for patients with very high triglycerides • Wants to speak about benefits for patients with persistently high triglycerides, as well as disseminate peer-reviewed literature – Seeks declaration that: • Application of FDA restrictions and FCA to speech about this particular off-label use, with the disclaimers Amarin proposed, would be unconstitutional as applied • With disclaimers, contends the speech is not misleading and thus subject to heightened scrutiny under Sorrell – Hearing on preliminary injunction scheduled for July 7

Hypothetical • Drug co. Meridia makes drug Merdia, approved in 2011. • Following a FOIA request to FDA, Meridia obtains the AE database relating to Merdia. – Based on this data, Meridia conducts a meta-analysis with a leading academic expert, and publishes its findings in an article in BMJ in 2015 showing the incidence rate of AEs associated with Merdia, which is < than contained in the PI. – The meta-analysis of this data also shows that Merdia increases HDL cholesterol, which is also included in BMJ article. ###
 Recent trends in ic engine
Recent trends in ic engine Foreign trade
Foreign trade Trends in project portfolio management
Trends in project portfolio management Mis trends
Mis trends Cpu output device
Cpu output device An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Halo syringe adaptor
Halo syringe adaptor Recent developments in ict
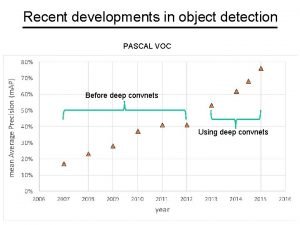
Recent developments in ict Recent developments in object detection
Recent developments in object detection Ap synthesis prompt
Ap synthesis prompt Advantages of scanning in reading
Advantages of scanning in reading Recent demographic changes in the uk
Recent demographic changes in the uk Myips portal
Myips portal Biotaphonomy
Biotaphonomy After miguel's recent automobile accident
After miguel's recent automobile accident A friend emails you the results of a recent high school
A friend emails you the results of a recent high school Recent advances in dental ceramics
Recent advances in dental ceramics News using passive voice
News using passive voice Http drive google com
Http drive google com Udin login
Udin login Comait
Comait A tagout device is preferable to using a lockout device.
A tagout device is preferable to using a lockout device. Kelompok input
Kelompok input Future trends in media and information
Future trends in media and information Body temperature maintenance
Body temperature maintenance Section 4 gene regulation and mutation
Section 4 gene regulation and mutation Rules and regulations in table tennis
Rules and regulations in table tennis Nutrition metabolism and body temperature regulation
Nutrition metabolism and body temperature regulation Recyclability and self regulation in nature
Recyclability and self regulation in nature Phosphoenolpyruvate
Phosphoenolpyruvate Nco duties and responsibilities
Nco duties and responsibilities Regulation of recruitment and placement activities
Regulation of recruitment and placement activities Section 4 gene regulation and mutation
Section 4 gene regulation and mutation Rules and regulation in table tennis
Rules and regulation in table tennis Regulation and inspection of social care wales
Regulation and inspection of social care wales Government regulation and the legal environment of business
Government regulation and the legal environment of business Wa department of water and environmental regulation
Wa department of water and environmental regulation Michigan department of licensing and regulation
Michigan department of licensing and regulation Licensing and regulation division
Licensing and regulation division