Quantum Monte Carlo And Weak Physical Interactions Ian


















![WHAT'S THE CONNECTION? THE ADIABATIC CONNECTION! _ Exc[n] = ½ n(r) nxc(r, r') dr WHAT'S THE CONNECTION? THE ADIABATIC CONNECTION! _ Exc[n] = ½ n(r) nxc(r, r') dr](https://slidetodoc.com/presentation_image_h/36e6adffbbf9cda06a6096ed2e6ba76b/image-29.jpg)





- Slides: 35

Quantum Monte Carlo And Weak Physical Interactions Ian Snook RMIT University, Applied Physics, School of Applied Sciences, SET Portfolio, GPO Box 2476 V, Melbourne, Victoria, Australia, 3001 1

COLLEAGUES OR THOSE WHO I BLAME FOR ERRORS/MISTAKES/MISCONCEPTIONS Dr Nicole Benedek Postdoctoral Fellow Imperial College Professor Richard Needs Mr Ryan Springall Ph. D Student RMIT Dr Mike Towler TCM Group, Cavendish Lab. , Cambridge Ass. Prof. Salvy Russo RMIT Dr Manolo Per Post. Doc RMIT 2

A LITTLE PERSPECTIVE 3

VICTORIA – SHOWING WINE REGIONS AND MELBOURNE 4

MELBOURNE AND RMIT APPLIED PHYSICS RMIT 5

MIKE OPENS NEW SPECIALTY SHOP IN MELBOURNE MIKE ADRESSES LARGE POLITICAL RALLY IN FEDERATION SQUARE 6

MIKE HUNTS FOR FOOD IN THE AUSSIE BUSH MIKE AT LAST FINDS HIS TRUE VOCATION 7

WHY BOTHER WITH van der Waals INTERACTIONS? THE ANSWER IS CONTAINED IN "PNAS September 17, 2002, 99 (19) 12252 -12256. Gecko Tanu Suryadi Kustadi (Nanyang Technical University, Singapore) “Wow for evolution. Weight by numbers. The ability of flies, beetles, skinks and geckos to stick to surfaces is in no way chemical, with bond strength being determined by the polarisabilty of the surface ( perturbation theory show the attractive potential between 2 atoms/molecules to be a function of the atomic polarisability of the 2 atoms) and the number of setae in contact with the surface, not being due to 8 suction or capillary forces [PNAS September 17,

WHAT ELSE? (THERE MUST BE MORE TO LIFE THAN GEKOS!) Interaction of Chemically Saturated Objects Biological Molecules Physical Adsorption Adhesion Surface-Surface Forces Initial Stages of Interactions with Surfaces vd. W Vd. W Universal at All Separations – Usually No Screening 9

WHY DO I WANT TO DO THIS? Or WHAT’S AN INNOCENT YOUNG AUSSIE DOIN’ ERE AMONGST YOU LOT? My Main Interests Are: • Statistical Mechanics of Condensed Matter • Surfaces • Interaction with Surfaces – Adsorption • Surface-Surface Interactions –Adhesion • Colloids and Nano-structures • Wine • Food Need at least a good Interaction Potential Energy Curve It would be nice if this were non-empirical N. B. CP-MD is Not Very Accurate for Many Systems Ds for H 2 O Liquid Off by Orders of Magnitude and vd. W Wrong 10

HOW STRONG IS WEAK ? • • These Interactions are a lot Weaker than Covalent, Ionic or Metallic Bonds Well Depth He 2 = 10 K = 0. 0008 au Well Depth Ar 2 = 160 K H 2 O – H 2 O De = 5 kcalmol-1 = 0. 004 au 11

WHAT METHODS ARE AVAILABLE? Quantum Chemical Methods CI MPn CC Problems: The Interaction Energy E is Obtained as a Difference EAB = EAB – (EA + EB) EAB (EA + EB) Variational Principle Does Not Apply to E Slow Convergence (e. g. MP 4 is not “exact” for He 2 or Ne 2 CC needs SD(T) for good results and SDT(Q) for “Exact” Results for He 2) Nm where m = 4 -9? ? Needs very Large Basis Set for the Non-HF Part (e. g. MP 4 for Ne 2 s, p, d Needed for HF but MP 4 s, p, d, f, g…. and/or Bond Functions*) Basis Set Superposition Error (BSSE) Large Need Counter Poise Correction (CPC) which Doubles the Calculation Bond Functions Suffer from “Molecular Bias”* G. Grochola, T. Petersen, S. P. Russo, I. K. Snook, Molecular Physics, 100 3867 -3872 (2003) 12

DFT Currently Available Approximate Functionals Do Not Describe van der Waals Interactions Correctly They are Somewhat Semi-empirical in Nature The Results for H-Bonds are Very Functional Dependent* BSSE is Still Important Even with Large Numerical Basis Sets Which are Available for H-bonds* * N. A. Benedek, I. K. Snook, K. Latham and I. Yarovsky, J. Chem. Phys. , 122, 144102 (2005) 13

Symmetry Adapted Perturbation Theory (SAPT) H = H(0) + V = HA + HB + HAB EAB = EAB – (EA + EB) = E(1) + E(2) + E(3) + …. R-S Perturbation Theory Positive Features Gets E Directly In Principle Exact for E Terms in E can be given a Physical Interpretation Negative Features n At least Third Order for Very Accurate Result n Don’t know anything about Convergence n The higher order terms become complicated n The energy expressions are not unique n Over-complete bases are used to get better results n Strictly need the exact ground state wavefunctions of the separated systems or Double Pert. Theory n Restricted to small systems – He 2 , (H 2 O)2 n In practice many different methods must be used to evaluate different parts of E - usually supermolecule plus SAPT 14

DFT = DEFINITELY FASHIONABLE THEORY ADVERTISING PITCH* AN ENDORSMENT FROM THE NOBEL PRIZE WINNING WALTER KOHN (“PROOF BY INSULT”): "WE CONCLUDE THAT TRADITIONAL WAVEFUNCTION METHODS, WHICH PROVIDE THE 'REQUIRED' CHEMICAL ACCURACY, ARE GENERALLY LIMITED TO MOLECULES WITH A SMALL TOTAL NUMBER OF CHEMICALLY ACTIVE ELECTRONS, N < O(10)“ AND "IN GENERAL THE MANY-ELECTRON WAVEFUNCTION (r 1, ……. , r. N) FOR A SYSTEM OF N ELECTRONS IS NOT A LEGITIMATE SCIENTIFIC CONCEPT, WHEN N N 0, WHERE N 0 103. " TAKEN FROM, W. KOHN, REV. MOD. PHYS. , 71 1257 (1998) • The management of this workshop does not necessarily endorse the products advertised by Professor Kohn 15

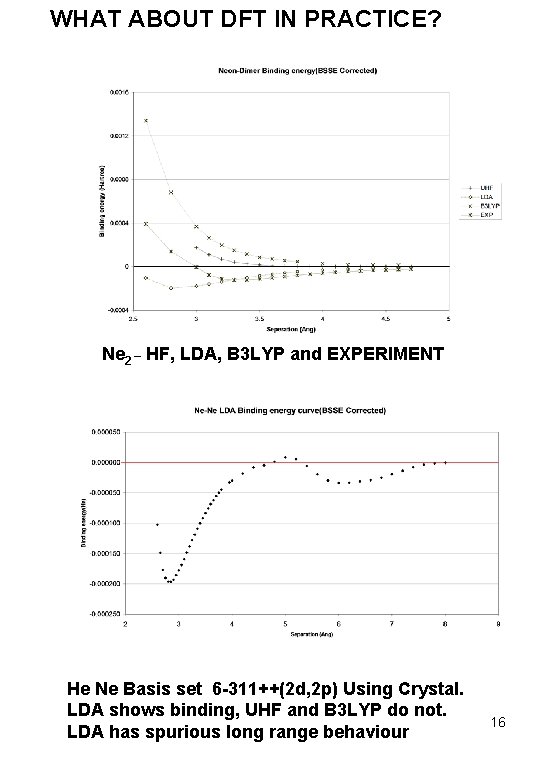
WHAT ABOUT DFT IN PRACTICE? Ne 2 _ HF, LDA, B 3 LYP and EXPERIMENT He Ne Basis set 6 -311++(2 d, 2 p) Using Crystal. LDA shows binding, UHF and B 3 LYP do not. LDA has spurious long range behaviour 16

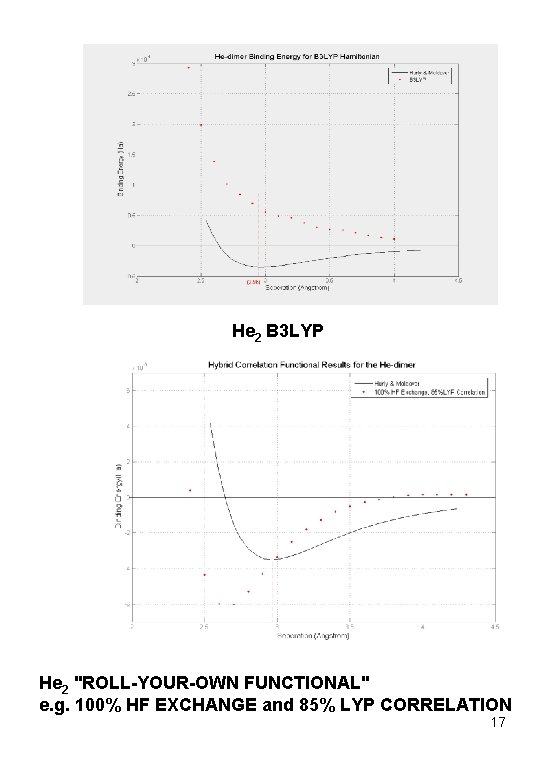
He 2 B 3 LYP He 2 "ROLL-YOUR-OWN FUNCTIONAL" e. g. 100% HF EXCHANGE and 85% LYP CORRELATION 17

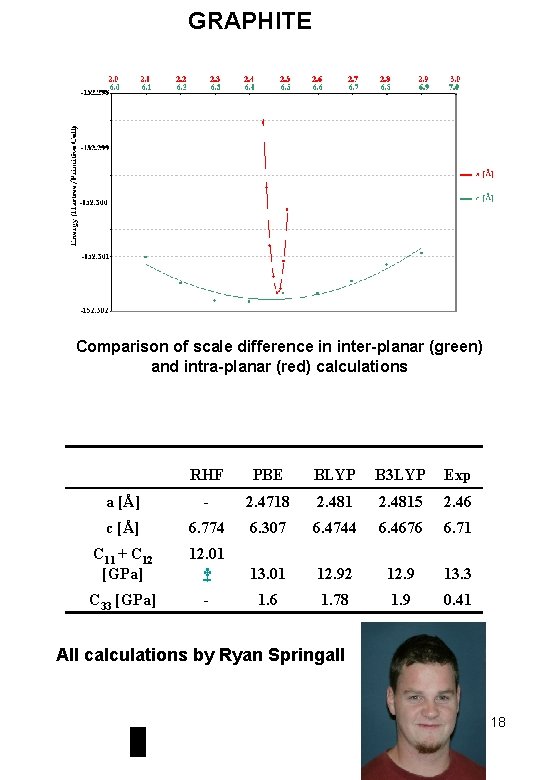
GRAPHITE Comparison of scale difference in inter-planar (green) and intra-planar (red) calculations RHF PBE BLYP B 3 LYP Exp a [Å] - 2. 4718 2. 4815 2. 46 c [Å] 6. 774 6. 307 6. 4744 6. 4676 6. 71 C 11 + C 12 [GPa] 12. 01 † 13. 01 12. 92 12. 9 13. 3 C 33 [GPa] - 1. 6 1. 78 1. 9 0. 41 All calculations by Ryan Springall 18

H-BONDS DFT using DMOL 3 and GAUSSIAN 03 GTO and Numerical Basis Sets BLYP, HCTH and PBE Functionals CPC with GTO’s Non with NBS N. A. Benedek, I. K. Snook, K. Latham and I. Yarovsky, J. Chem. Phys. , 19 122 144102 (2005)

DFT = DEAD F#@$’ING THEORY? ? • OBITUARY: DENSITY FUNCTIONAL THEORY (1927 -1993)", PETER M. W. GILL, AUST. J. CHEM. 54 661 -662 (2001) • TO QUOTE " SHE WAS MISUNDERSTOOD AND ABUSED, HELD IN NAÏVE AWE BY SOME, AND IN CONTEMPT BY OTHERS, CAPABLE OF STUNNING SUCCESSES AND DISMAL FAILURES. HER SIMPLICITY WAS SEDUCTIVE BUT HER FLAWS RAN DEEP AND, IN THE END, HER FALL WAS INEVITABLE“ RIP 20

WHAT NOW? TRY TO RE-HABILITATE WAVEFUNCTIONS AND RID THEM OF THEIR EXCESSES (Nm !) TRY TO RE-INCARNATE DFT – HOW? BECOME A GAMBLER ENTER THE CASINO! – A PARTICULARLY AUSTRALIAN RESPONSE TO LIFE'S PROBLEMS I FEAR 21

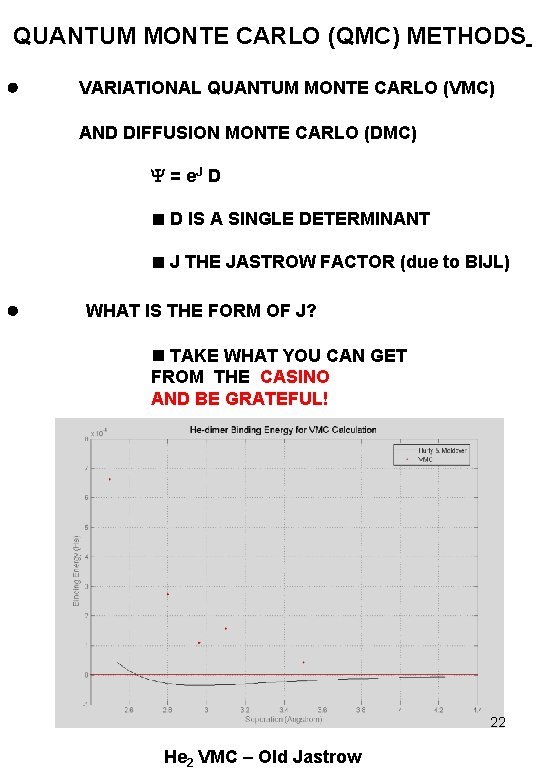
QUANTUM MONTE CARLO (QMC) METHODS VARIATIONAL QUANTUM MONTE CARLO (VMC) AND DIFFUSION MONTE CARLO (DMC) = e. J D D IS A SINGLE DETERMINANT J THE JASTROW FACTOR (due to BIJL) WHAT IS THE FORM OF J? TAKE WHAT YOU CAN GET FROM THE CASINO AND BE GRATEFUL! 22 He 2 VMC – Old Jastrow

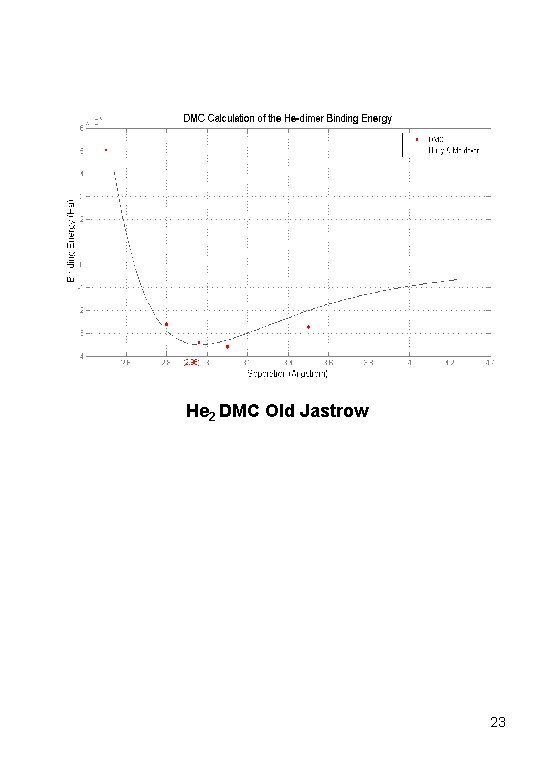
He 2 DMC Old Jastrow 23

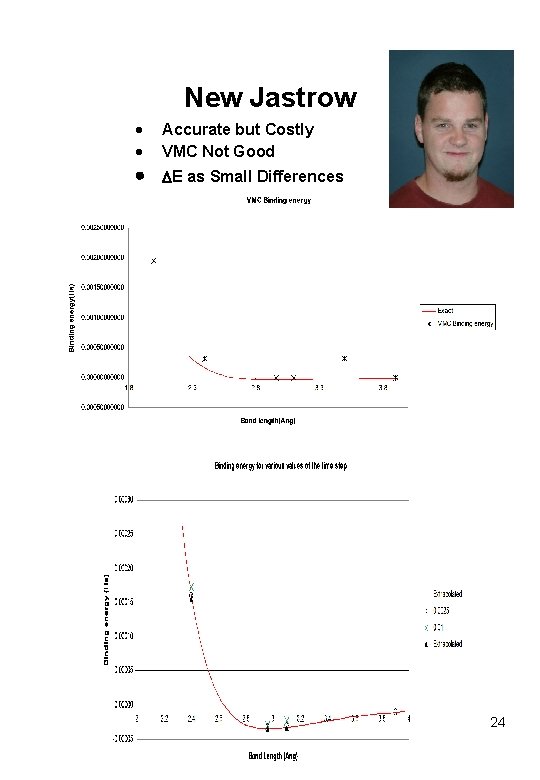
New Jastrow Accurate but Costly VMC Not Good E as Small Differences 24

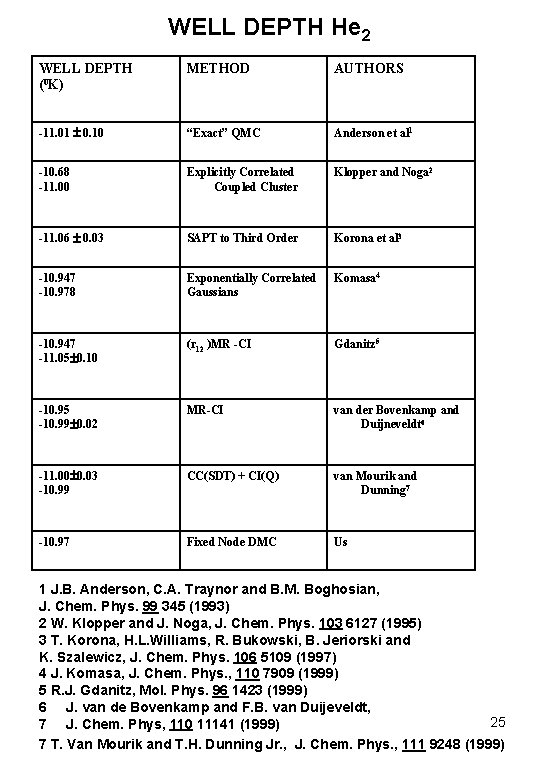
WELL DEPTH He 2 WELL DEPTH (0 K) METHOD AUTHORS -11. 01 0. 10 “Exact” QMC Anderson et al 1 -10. 68 -11. 00 Explicitly Correlated Coupled Cluster Klopper and Noga 2 -11. 06 0. 03 SAPT to Third Order Korona et al 3 -10. 947 -10. 978 Exponentially Correlated Gaussians Komasa 4 -10. 947 -11. 05 0. 10 (r 12 )MR -CI Gdanitz 5 -10. 99 0. 02 MR-CI van der Bovenkamp and Duijneveldt 6 -11. 00 0. 03 -10. 99 CC(SDT) + CI(Q) van Mourik and Dunning 7 -10. 97 Fixed Node DMC Us 1 J. B. Anderson, C. A. Traynor and B. M. Boghosian, J. Chem. Phys. 99 345 (1993) 2 W. Klopper and J. Noga, J. Chem. Phys. 103 6127 (1995) 3 T. Korona, H. L. Williams, R. Bukowski, B. Jeriorski and K. Szalewicz, J. Chem. Phys. 106 5109 (1997) 4 J. Komasa, J. Chem. Phys. , 110 7909 (1999) 5 R. J. Gdanitz, Mol. Phys. 96 1423 (1999) 6 J. van de Bovenkamp and F. B. van Duijeveldt, 25 7 J. Chem. Phys, 110 11141 (1999) 7 T. Van Mourik and T. H. Dunning Jr. , J. Chem. Phys. , 111 9248 (1999)

H 2 O – H 2 O VMC and DMC using CASINO Single Determinant Plus Jastrow Large Basis Set Extrapolate to t = 0 All Electron Orbitals from HF and B 3 LYP 26

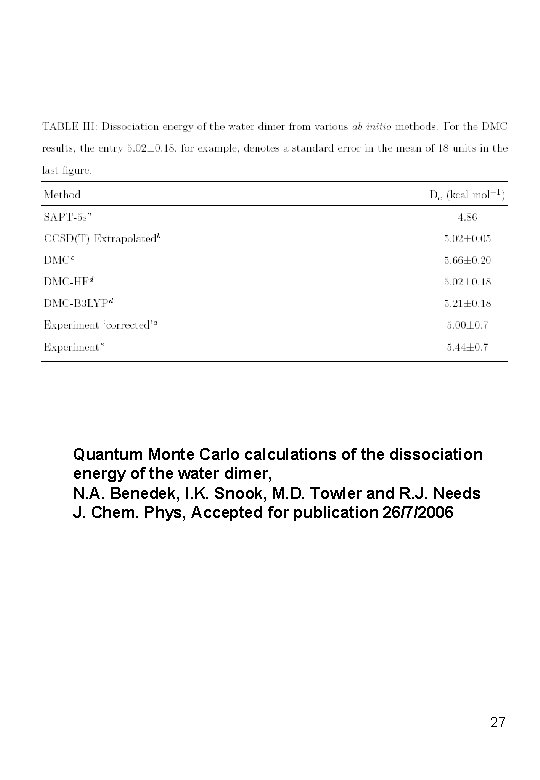
Quantum Monte Carlo calculations of the dissociation energy of the water dimer, N. A. Benedek, I. K. Snook, M. D. Towler and R. J. Needs J. Chem. Phys, Accepted for publication 26/7/2006 27

CAN WE RE-INCARNATE DFT TO MAKE IT A “DEFINITELY FINE THEORY” ? It would be valuable to be able to find the form of exchange-correlation functional for some particular van der Waals systems Use this to aid in constructing better approximate forms of this functional This can to be done, in principle, by Quantum Monte Carlo (QMC) Calculate the exact exchange-correlation energy density and exchange-correlation hole function 28
![WHATS THE CONNECTION THE ADIABATIC CONNECTION Excn ½ nr nxcr r dr WHAT'S THE CONNECTION? THE ADIABATIC CONNECTION! _ Exc[n] = ½ n(r) nxc(r, r') dr](https://slidetodoc.com/presentation_image_h/36e6adffbbf9cda06a6096ed2e6ba76b/image-29.jpg)
WHAT'S THE CONNECTION? THE ADIABATIC CONNECTION! _ Exc[n] = ½ n(r) nxc(r, r') dr dr' r - r' where _ 1 nxc(r, r') = nxc(r, r')d 0 nxc(r, r') is the exchange-correlation hole of a fictitious system in which the strength of the electron-electron interaction has been reduced by a factor while the external potential has been adjusted to keep the electron density at n(r). This requires the use of a Hamiltonian operator defined by H = T + V We may also define the exchange-correlation energy density by _ exc(r) = ½ n(r) nxc(r, r') dr' r - r' so that Exc[n] = exc(r) dr 29

RELATION TO PDF _ Now nxc(r, r') is related to the coupling-constant_ integrated pair correlation function g(r, r') by _ _ nxc(r, r') = n(r)[ g(r, r') – 1] _ If we write g in terms of its constituent spin components i. e. _ _ g(r, r') = [n (r)n (r')/ n (r)n (r')] g (r, r') then _ g (r, r') = [N(N-1)/ n (r)n (r')] 1 x d dx 3 ……dx. N (r , r' , x 3 ……, x. N) 2 0 where N is the number of electrons, n (r) is the electronic density for spin and xi is the ith electron's spatial and spin co-ordinates. All this can be done by VMC. 30

WHAT ARE WE UP TO? He 3 at Short Distances to Describe Very High Pressure He Melting He 3 Larger Separations by SAPT PMIC Phase Diagram of He 4 from First Principles Better Jastrows for vd. W Adiabatic Connection Method Seeing if DMC can be used with Adiabatic Connection 31

Acknowledgment RMIT Supercomputer Centre 32

• Good Bye and Thank You • Kindly Direct Any Awkward Questions to Mike Towler and/or Richard Needs 33

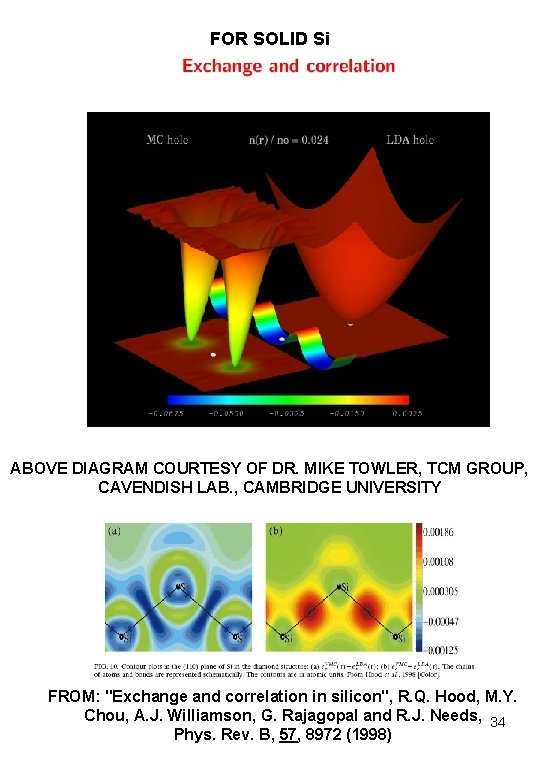
FOR SOLID Si ABOVE DIAGRAM COURTESY OF DR. MIKE TOWLER, TCM GROUP, CAVENDISH LAB. , CAMBRIDGE UNIVERSITY FROM: "Exchange and correlation in silicon", R. Q. Hood, M. Y. Chou, A. J. Williamson, G. Rajagopal and R. J. Needs, 34 Phys. Rev. B, 57, 8972 (1998)

STRONGLY INHOMOGENEOUS ELECTRON GAS FROM: "Quantum Monte Carlo Analysis of Exchange and Correlation in the Strongly Inhomogeneous Electron Gas", M. Nekovee, W. M. C. Foulkes and R. J. Needs, Phys. Rev. Letters, 87, 36401 (2001) 35