Proximate Analysis q Definition Proximate Analysis is a




























- Slides: 46

Proximate Analysis

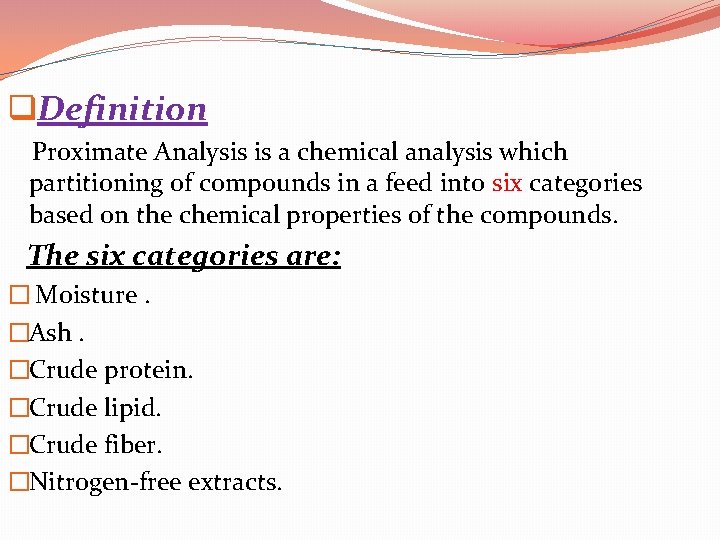
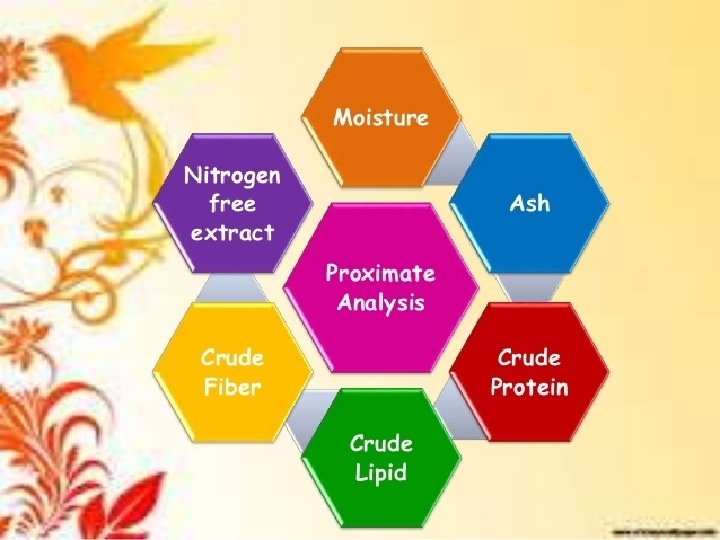
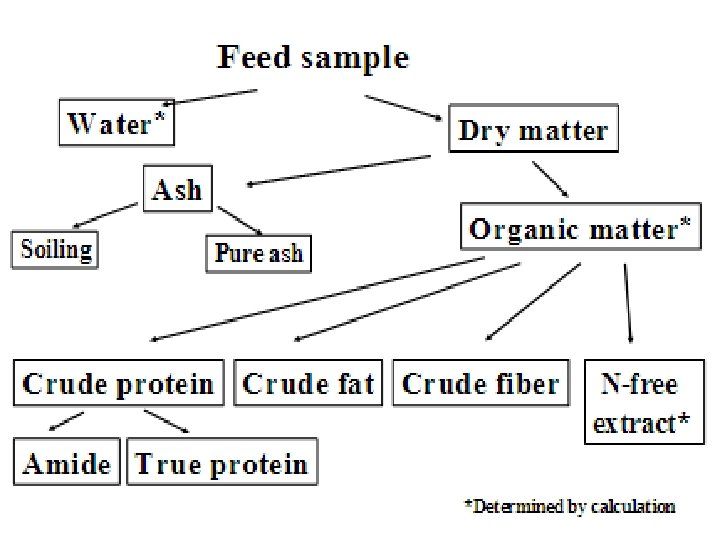
q. Definition Proximate Analysis is a chemical analysis which partitioning of compounds in a feed into six categories based on the chemical properties of the compounds. The six categories are: � Moisture. �Ash. �Crude protein. �Crude lipid. �Crude fiber. �Nitrogen-free extracts.



Moisture Ø Moisture content is an important factor in both economy & storage. Ø At high temperature & high humidity in Egypt ( more than 15 % ) moisture : � Risk of growth of molds. � Self digestion by enzymes. Ø Over 8% insects. Ø Over 14% fungi & bacteria.

�How to determine water content ? ? ? By drying sample in an oven at 105 c , 12 hours. Water= Sample weight – Dry matter weight

Volatile Matter q. Definition: � Unstable material Transform from one form to another ( turn into a gas or vapor). �Has low boiling point. q. Examples : Nitrogen , H 2 O , CO 2 , ammonia and hydrogen. �Feed contain ammonia and flavor components Which vaporized & hardly measured.

q. Moisture Equilibrium �Process of hygroscopic material A substance tending to absorb moisture from the air to reach equilibrium with humidity of air by absorbing or desorbing moisture. �When feed spread on flat sheet and left , moisture absorbing or release to reach equilibrium. �Relative humidity in Egypt is 70%. �Moisture content in equilibrium is 13 -15%.

Dry matter Determination of dry matter: 1. Determination of both water and dry matter. 2. Moisture essay method. 3. Distillation. 4. Karl Fischer method. 5. Heating furnace control method. 6. Other methods.

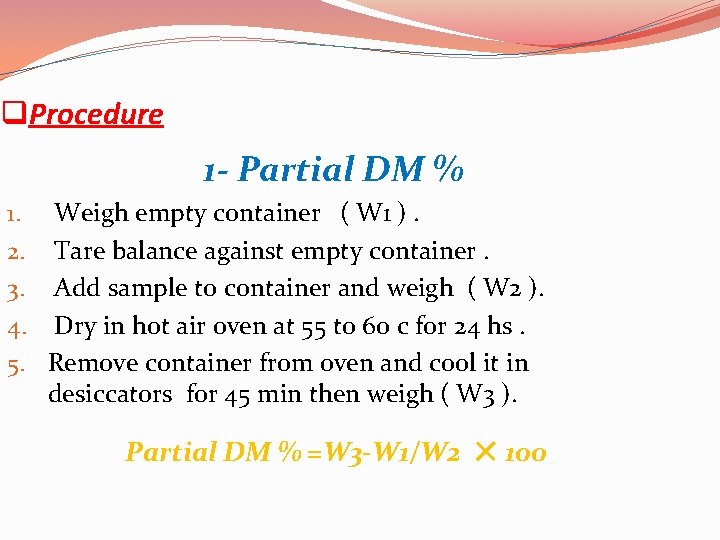
1 - Determination Of Both Water And Dry Matter This procedure is used to determine the DM% of all feed stuff except silage. q Principle : 1 - Partial DM % Wet sample ( < 85% DM ) is dried at 55 to 60 c in air oven to reduce moisture content for 24 hours before grinding .

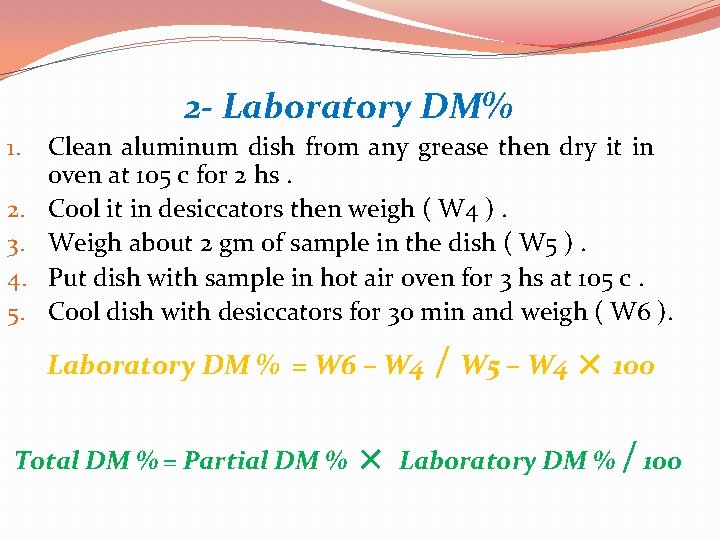
2 - Laboratory DM% � Sample is finely grounded and dried at 105 c in air oven for 2 -4 hours. � Equipments used 1. 2. 3. 4. 5. 6. 7. Forced hot air oven. Digital balance. Containers to hold samples. Aluminum dishes. Desiccators. Forceps. Recording sheet or computer .

Forced Hot Air Oven Digital Balance

Aluminum Dish Glass Dish

Desiccators

q. Procedure 1 - Partial DM % 1. 2. 3. 4. 5. Weigh empty container ( W 1 ). Tare balance against empty container. Add sample to container and weigh ( W 2 ). Dry in hot air oven at 55 to 60 c for 24 hs. Remove container from oven and cool it in desiccators for 45 min then weigh ( W 3 ). Partial DM % =W 3 -W 1/W 2 × 100

2 - Laboratory DM% 1. 2. 3. 4. 5. Clean aluminum dish from any grease then dry it in oven at 105 c for 2 hs. Cool it in desiccators then weigh ( W 4 ). Weigh about 2 gm of sample in the dish ( W 5 ). Put dish with sample in hot air oven for 3 hs at 105 c. Cool dish with desiccators for 30 min and weigh ( W 6 ). Laboratory DM % = W 6 – W 4 Total DM % = Partial DM % × / W 5 – W 4 × 100 Laboratory DM % / 100

Notes Aluminum dish more used than glass dish because : 1. Less fragile. 2. Lighter. 3. Better thermal conductivity. 4. Easy to handle.

Desiccant substances : 1. Silica gel. 2. Calcium chloride. 3. Phosphorus pentoxide. Silica gel commonly used due to : 1. Easy to handle. 2. Regenerate.

2 - Distillation �Used for a sample With high content of volatile components such as silage. �Procedure 1. Heat a sample with an organic solvent as toluene or xylene or tetrachlorethylene which is immiscible with water. 2. Water is distilled from sample and trapped under layer of toluene. 3. Cool it & calculate water in sample which separated from solvent.

3 - Karl Fischer method �Used for food with very low moisture content and for sample contain volatile component. �Karl Fischer reagent : 1. Iodine. 2. Sulfur dioxide. 3. Pyridine. 4. Methanol.

4 - Other method Physical Removal Of Water : 1. 2. 3. 4. 5. Hot air oven. Vacuum oven. Freeze drying. Microwave oven. Dean & stark distillation.

Chemical method 1. Karl Fischer method. physical method 1. NMR Nuclear magnetic resonance. 2. NIR Near infrared reflectance. Chromatography 1. GLC Gas liquid chromatography. 2. GSC Gas solid chromatography.

Determination Of Crude Protein

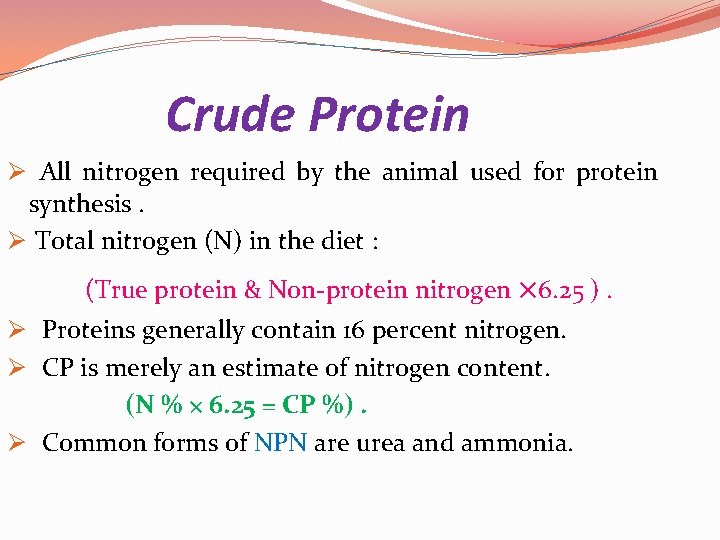
Crude Protein Ø All nitrogen required by the animal used for protein synthesis. Ø Total nitrogen (N) in the diet : (True protein & Non-protein nitrogen × 6. 25 ). Ø Proteins generally contain 16 percent nitrogen. Ø CP is merely an estimate of nitrogen content. (N % × 6. 25 = CP %). Ø Common forms of NPN are urea and ammonia.

q. Sample Preparation 1. Solid foods are ground to pass a 20 -mesh screen. 2. Samples for analysis should be homogeneous.

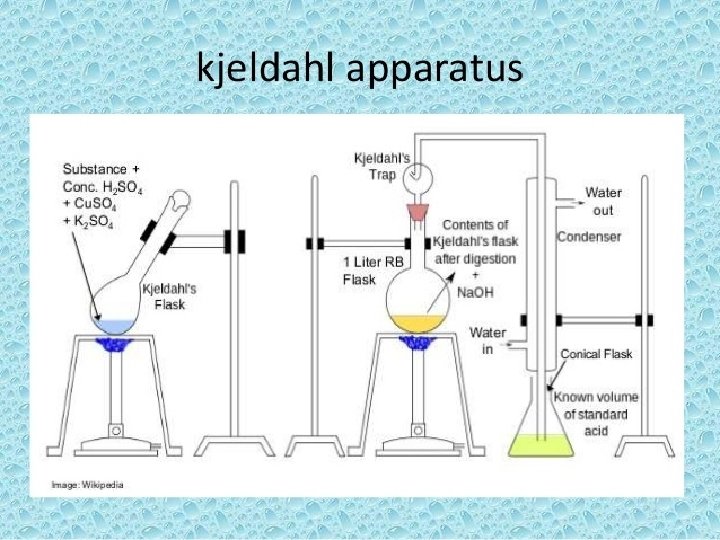
Kjeldahl Method q. Principle �Proteins and other organic food components in a sample are digested with sulfuric acid in the presence of catalysts. �The total organic nitrogen is converted to ammonium sulfate. �The digest is neutralized with alkali and distilled in boric acid solution. �The borate anions formed are titrated with standardized acid, which is converted to nitrogen in the sample. �The result of the analysis represents the crude protein content of the food since nitrogen also comes from non protein components.

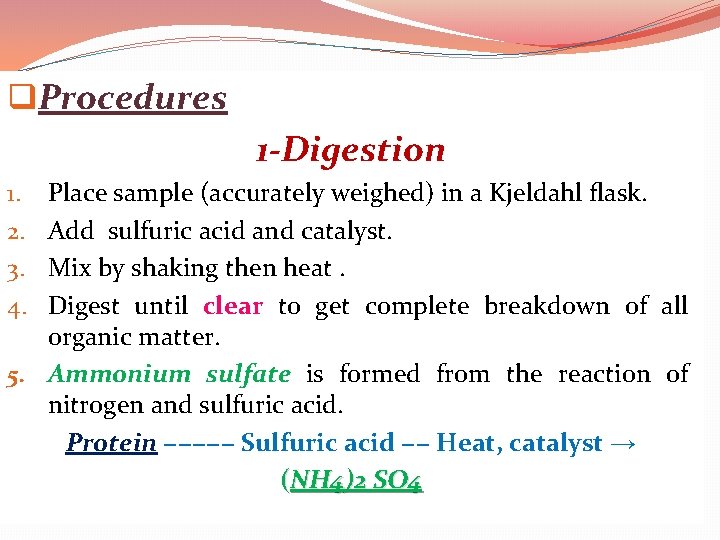
q. Procedures 1 -Digestion Place sample (accurately weighed) in a Kjeldahl flask. Add sulfuric acid and catalyst. Mix by shaking then heat. Digest until clear to get complete breakdown of all organic matter. 5. Ammonium sulfate is formed from the reaction of nitrogen and sulfuric acid. Protein −−−−− Sulfuric acid −− Heat, catalyst → (NH 4)2 SO 4 1. 2. 3. 4.


Kjeldahl apparatus Digestion Unit

Notes �Potassium sulphate: Is an oxidizing agent used to elevate boiling point. �Copper sulphate : Is a catalyst used to facilitate degradation. �To stop foaming use : 1. Paraffin. 2. Pumic stones. 3. Granulated zinc. � Ammonia may be lost when : 1. Increasing heat temperature. 2. Decreasing solution volume after degradation.

3 -Distillation �The digest is diluted with water. � Alkali-containing sodium hydroxide is added to neutralize the sulfuric acid. ( NH 4)2 SO 4 +2 Na. OH→ 2 NH 3 +Na 2 SO 4 +2 H 2 O �The ammonia formed is distilled into sulfuric acid or boric acid solution containing the indicators methylene blue and methyl red. End point : When red purple color is turned to blue color then to green color.

Distillation unit


4 -Titration Goal : Indicate the amount of ammonia present in the distillate. Two types: 1. Back titration. 2. Direct titration.

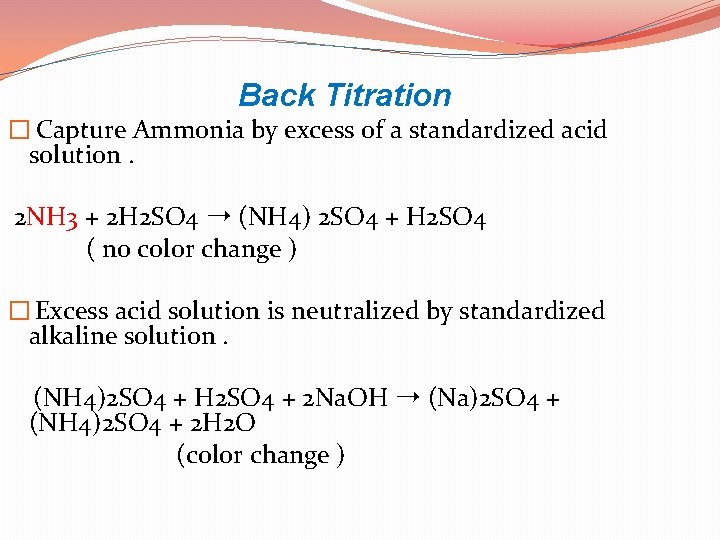
Back Titration � Capture Ammonia by excess of a standardized acid solution. 2 NH 3 + 2 H 2 SO 4 ➝ (NH 4) 2 SO 4 + H 2 SO 4 ( no color change ) � Excess acid solution is neutralized by standardized alkaline solution. (NH 4)2 SO 4 + H 2 SO 4 + 2 Na. OH ➝ (Na)2 SO 4 + (NH 4)2 SO 4 + 2 H 2 O (color change )

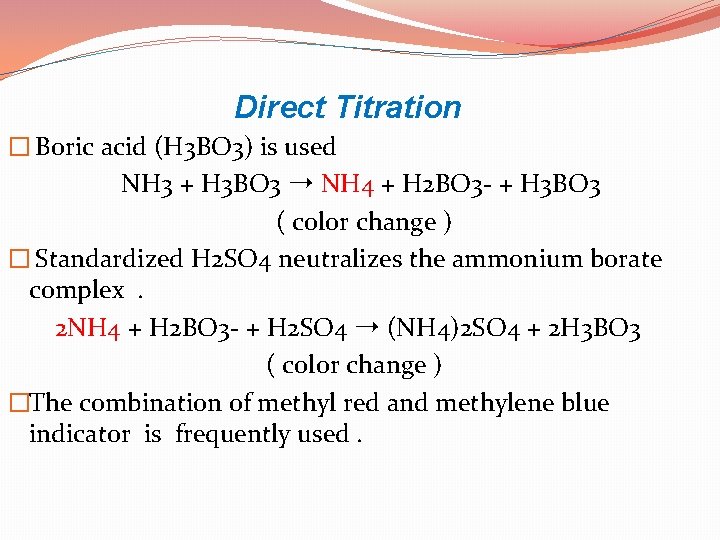
Direct Titration � Boric acid (H 3 BO 3) is used NH 3 + H 3 BO 3 ➝ NH 4 + H 2 BO 3 - + H 3 BO 3 ( color change ) � Standardized H 2 SO 4 neutralizes the ammonium borate complex . 2 NH 4 + H 2 BO 3 - + H 2 SO 4 ➝ (NH 4)2 SO 4 + 2 H 3 BO 3 ( color change ) �The combination of methyl red and methylene blue indicator is frequently used.



5 - Calculation �When boric acid is used as the receiving solution the equation is : Direct Titration % nitrogen = (ml standard acid x M of acid ) x 14. 007 X 100 weight of sample in grams �When standard acid is used as the receiving solution the equation is: Back Titration % nitrogen= (ml standard acid x M of acid)- (ml standard base x M of base) x 14. 007 x 100 weight of sample in grams

Kjeldahl Apparatus

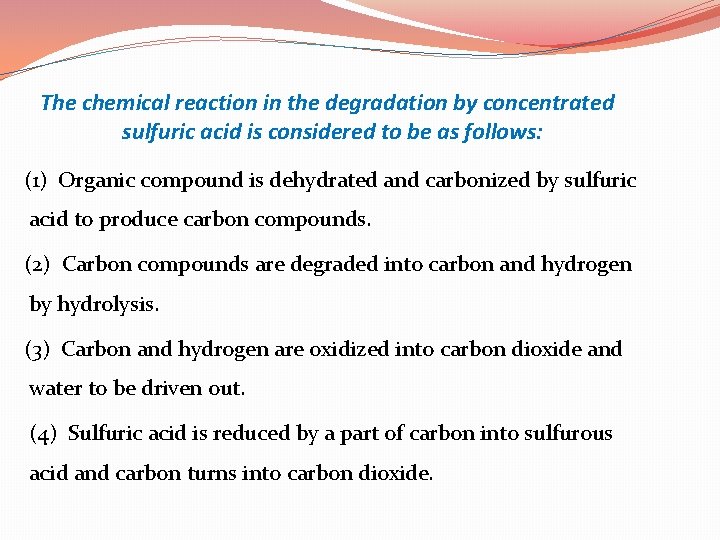
The chemical reaction in the degradation by concentrated sulfuric acid is considered to be as follows: (1) Organic compound is dehydrated and carbonized by sulfuric acid to produce carbon compounds. (2) Carbon compounds are degraded into carbon and hydrogen by hydrolysis. (3) Carbon and hydrogen are oxidized into carbon dioxide and water to be driven out. (4) Sulfuric acid is reduced by a part of carbon into sulfurous acid and carbon turns into carbon dioxide.

(5) Sulfurous acid reduces nitrogen into ammonia, and itself turns into sulfuric anhydride. (6) Hydrogen produced by the degradation of carbon compounds facilitates the formation of ammonia. (7) Ammonia formed immediately combines with sulfuric acid to become ammonium sulfate. ( 8) When potassium sulfate is added as an oxidizing agent, the boiling point is elevated and the effect on organic compound becomes more active.

Combustion Method Or Dumas Method Ø 1. 2. 3. 4. Advantages : Rapid quantification. No druft ( no need to degrade sample ). Environmental load is smaller. Easily operated. Ø Tow method used in : 1. Fertilizer analysis. 2. Food analysis. 3. Plant analysis.

Methods Of Nitrogen Analysis : Total nitrogen 1. Kjeldahl method. 2. Dumas method. 3. Radiochemical method. Analysis with automated instrument 1. Super kjel automatic nitrogen. 2. Kjeltec auto system. 3. Kjeldahl method.

Methods applicable to specific food : 1. 2. 3. 4. 5. 6. Formol titration. Biuret. Folins reagent. Alkaline distillation. Dye binding. Near infrared reflectance.
