WELCOME ELEMENTARY QUANTITATIVE ANALYSIS CHEM 116 ELEMENTARY QUANTITATIVE















- Slides: 27

WELCOME! ELEMENTARY QUANTITATIVE ANALYSIS CHEM 116

ELEMENTARY QUANTITATIVE ANALYSIS CHEM 116, Spring 2009 Tues & Thur 12: 30 -1: 20, Rm. 112 Hamilton Hall COURSE OUTLINE Instructor: Dr. Robert Powers Office Address: 722 Ha. H Phone: 472 -3039 e-mail: rpowers 3@unl. edu web page: http: //bionmr-c 1. unl. edu/ Labs 720 -721 Ha. H 472 -5316 Office Hours: 10: 30 -11: 30 am TR or by Special Appointment I am in my office many other times during the week and am always willing to speak with you if you find me in or make an appointment. Teaching Assistants: Ms. Jennifer Copeland Mr. Roberto Delgadillo phone: 472 -5316 phone: 472 -1430 office: 721 Ha. H office: 738 Ha. H

COURSE OUTLINE Required Items: (i) Chem. 113 is a prerequisite. (ii) Are you registered for Chem. 114? (iii) Text: "Quantitative Analysis" 7/e Daniel C. Harris, Freeman & Co. , New York (iv) Lab Manual: "Laboratory Manual for Quantitative Chemical Analysis", J. D. Carr (2007) (v) Laboratory Notebook: bound (not spiral), use one with grids for graphs. (vi)Padlock for lab station and lab deposit card (vii) Black Sharpie for labeling glassware (viii) Calculator for exams and lab (TI-89 style or a simpler model) (ix) Laptop (optional) to run Excel calculations during lab Course Work: Midterm Exam: Final: Laboratory: Lab Notebook: Total: 100 pts 250 pts 600 pts (Thurs. , March 12) (10 am-12 pm, Fri. , May 8) (due at end of each lab) (due during lab check-out) Homework problem sets will not be collected or graded, but will aid your preparation for the exams. Grading scale: A+=95%; A=90%; A-=85%; B+=80%; B=75%; B-=70%; C+=65%; C=60%; C-=55%; D=50%; D-=45%; F=40%

Lecture Topics Date Chapter Topic Problems Jan 13 Jan 15 Jan 20 Jan 22 Jan 27 Jan 29 Feb 3 Feb 5 Feb 10 Feb 12 Feb 17 Feb 19 Feb 24 Feb 26 Mar 3 Mar 5 Mar 10 Mar 12 Mar 16 -20 Mar 24 Mar 26 Mar 31 Apr 2 Apr 7 Apr 9 Apr 14 Apr 16 Apr 21 Apr 23 Apr 28 Apr 30 May 8 Chap 0 & 1 Chap 2 & 3 Chap 27 Chap 4 Chap 5 Chap 6 Chap 7 Chap 8 Chap 12 Measurement Tools Error Gravimetry Statistics (cont) Calibration Equilibrium (Intro) Equilibrium (cont) Titrations (cont) Activity Equilibrium (systematic) Equilibrium (cont) Equilibrium (even more) EDTA Titrations (cont) MIDTERM EXAM Fall Break Electrochemistry Redox Titrations Spectrophotometry (cont) Monoprotic acid/base (cont) Separations Gas Chromatography (cont) Polyprotic acid/base Acid/base Titrations (cont) FINAL EXAM 10: 00 -12: 00 0 -1, 5 -A, 6 & 1 -5, 7, 22, 24, 26 2 -D, 1, 10, 15, & 3 -A, 5, 9, 11 3 -12, 13, 15, 18, 21, 23 27 -2, 3, 7, 14, 18, 25, 26 4 -B, E, 2, 3, 6 4 -9, 11, 13, 14, 15, 18, 22 5 -A, B, C, 22, 23 6 -A, B, G, I, K, 1, 2, 3, 5, 13 6 -17, 21, 37, 40, 54 7 -B, C, D, 1, 2, 4, 8, 11, 13 Chap 14 Chap 16 Chap 18 Chap 9 Chap 23 Chap 24 Chap 10 Chap 11 8 -A, C, 1, 4, 8, 14 8 -F, G, H, 10, 16 8 -18, 8 -21, 8 -23 8 -26, 28 12 -B, 2, 4, 5, 12 -6, 13, 22, 28 14 -B, D, I, 2, 3, 15, 18, 25, 41 16 -A, C, 1, 2, 7, 14, 15, 16, 24 18 -A, C, D, 1, 6, 8, 16, 18, 19 9 -B, C, G, H, 4, 6, 10, 13, 19 9 -24, 26, 27, 29, 30, 36, 37 23 -B, 1, 2, 3, 29, 44 24 -A, B, C 10 -A, 1, 2, 4, 7, 9, 16, 23, 29, 31, 33, 38 11 -A, B, F, G, I, 3, 5, 6, 7, 13, 16 11 -23, 27, 34, 36, 45, 46, 54, 64

Tentative Lab Schedule Date Experiment Value Jan 12 -16 Check-in, Safety, Introduction to Analytical Chemistry (Exp 1) 10 Jan 20 -23 Statistical Treatment of Data (Exp 2) 15 Jan 26 -30 Gravimetric Determination of Aluminum (Exp 3) 35 Feb 2 -6 Gravimetric Determination of Aluminum (cont) Feb 9 -13 Volumetric Determination of Soda Ash (Exp 4) Feb 16 -20 Volumetric Determination of Soda Ash (cont) Feb 23 -27 Determination of the Purity and p. Ka of Weak Organic acid (Exp 5) 30 Mar 2 -6 Determination of the Purity and p. Ka of Weak Organic acid (cont) 25 Mar 9 -13 Water Hardness by EDTA Titration (Exp 6) 25 Mar 23 -27 Coulometric Determination of Vitamin C (Exp 14 ) 25 Mar 30 -Apr 3 Spectrophotometric Determination of Iron (Exp 11 ) 25 Apr 6 -10 Potentiometric Titration of Iron (Exp 7 ) 25 Apr 13 -17 Gas chromatographic Analysis of Aromatic Hydrocarbons (Exp 15 ) 25 Apr 20 -24 Lab Make-up Apr 27 – May 1 Check out of lab 40 TOTAL 250 NOTE: Students having a Monday lab should make up Jan 19 lab on Jan 23 (Friday)

COURSE OUTLINE Lectures: ALL Power. Point lecture notes are available online on Black. Board and my website (http: //bionmr-c 1. unl. edu/). !!!!The Lectures Notes Are Not Meant To Replace Attending Class!!!!! Laboratory: 50% of your grade in CHEM 116 is based on your laboratory effort. Ø You will be furnished samples whose composition is unknown to you. Ø You will be asked to determine how much of a given analyte is present. Ø You will be graded on how well you agree with the correct answer. Ø You are allowed to re-do one lab during the next to last week § You are allowed to re-submit lab calculations if you made a math mistake at no: § you must clearly state what the mistake was and how you corrected it q no penalty for first re-submission q 15% penalty for each subsequent resubmission of a calculation error q Ø You will also be graded on how well you keep and maintain your lab notebook. Good Lab Practice and Techniques are Essential

Lab Notebook Techniques § The Lab Notebook Must: State what was done. Ø State what was observed Ø Be understandable to someone else Ø § Include Complete Description of Experiment: Purpose Ø Methods Ø Results Ø Conclusions Ø § Include Balanced Chemical Equations for Every Reaction Used § Paste Hardcopies of Important Data in Notebook § Include Calculations, Titles, Dates and Table of Contents § Notebooks are Legal Documents and Routinely Used for Patent Litigation § Laboratory Notebook should be bound (not spiral), use one with grids instead of lined pages for graphs.

Lab Notebook Techniques This Notebook Page is incomplete and a Useless Document. Limited Detail. This Notebook Page Has Precise Description with Adequate Detail

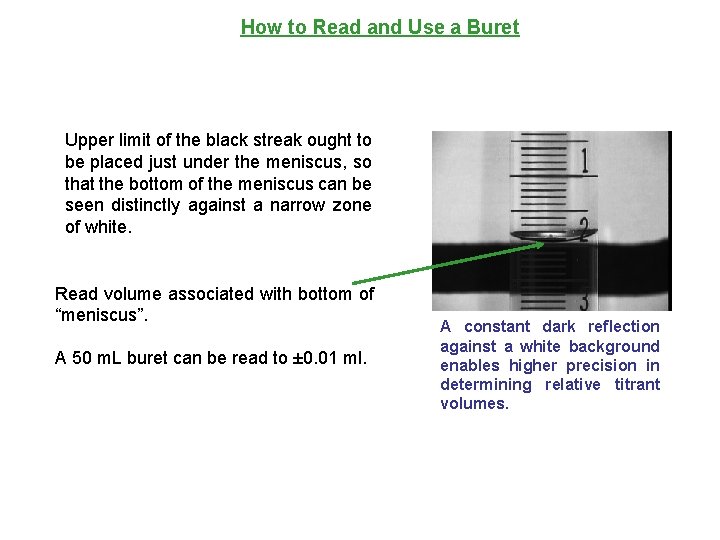
How to Read and Use a Buret When reading a buret, it is important that your line of sight be in a direction perpendicular to the buret column. All buret reading should be done using a buret card.

How to Read and Use a Buret Upper limit of the black streak ought to be placed just under the meniscus, so that the bottom of the meniscus can be seen distinctly against a narrow zone of white. Read volume associated with bottom of “meniscus”. A 50 m. L buret can be read to ± 0. 01 ml. A constant dark reflection against a white background enables higher precision in determining relative titrant volumes.

How to Read and Use a Buret A bubble in the nozzle of a buret will produce an inaccurate volume reading if the bubble escapes during a titration The quickest way to get rid of bubbles is to fill the buret with titrant and open the valve. Some bubbles may require “light” tapping to dislodge them.

Microsoft Excel Demo

Introduction to Analytical Chemistry Identifying an Unknown Is Not As Easy as Portrayed by the CSI TV Show. Typically Requires More Than One Experiment and > 45 Minutes of Analysis with corresponding high cost (single DNA analysis ~$10, 000) CSI: Crime Scene Investigation

Introduction to Analytical Chemistry Background 1. ) Definition: ANALYTICAL CHEMISTRY: The Science of Chemical Measurements. 2. ) Types of Questions Asked in Analytical Chemistry a. ) What is in the sample? (qualitative analysis) b. ) How much is in the sample? (quantitative analysis) 3. ) Techniques used in Analytical Chemistry: a. ) Wet Chemical Methods: titrations, color-forming reactions, precipitations, etc. b. ) Instrumental Methods: spectrometry, chromatography, etc. What is it ? How much is there? How pure is it? What are the impurities?

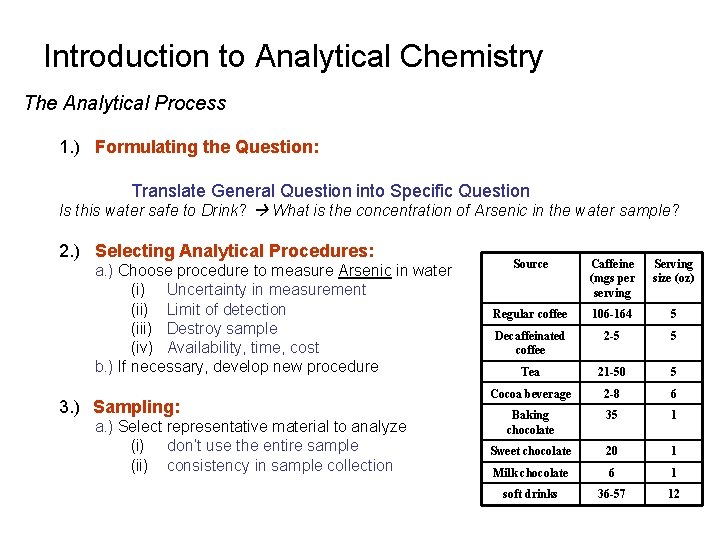
Introduction to Analytical Chemistry The Analytical Process 1. ) Formulating the Question: Translate General Question into Specific Question Is this water safe to Drink? What is the concentration of Arsenic in the water sample? 2. ) Selecting Analytical Procedures: a. ) Choose procedure to measure Arsenic in water (i) Uncertainty in measurement (ii) Limit of detection (iii) Destroy sample (iv) Availability, time, cost b. ) If necessary, develop new procedure 3. ) Sampling: a. ) Select representative material to analyze (i) don’t use the entire sample (ii) consistency in sample collection Source Caffeine (mgs per serving Serving size (oz) Regular coffee 106 -164 5 Decaffeinated coffee 2 -5 5 Tea 21 -50 5 Cocoa beverage 2 -8 6 Baking chocolate 35 1 Sweet chocolate 20 1 Milk chocolate 6 1 soft drinks 36 -57 12

Introduction to Analytical Chemistry The Analytical Process 4. ) Sample Preparation: a. ) convert sample into form suitable for chemical analysis (i) Dissolve sample (ii) Concentrate sample (iii) Remove species that interfere with analysis

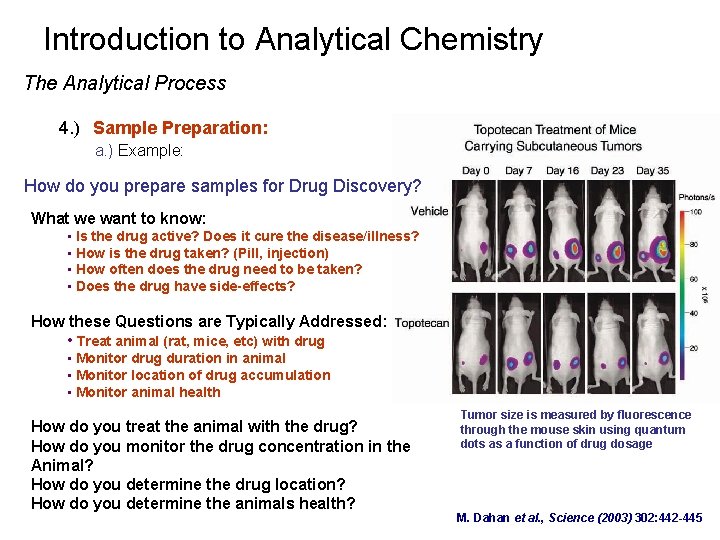
Introduction to Analytical Chemistry The Analytical Process 4. ) Sample Preparation: a. ) Example: How do you prepare samples for Drug Discovery? What we want to know: • Is the drug active? Does it cure the disease/illness? • How is the drug taken? (Pill, injection) • How often does the drug need to be taken? • Does the drug have side-effects? How these Questions are Typically Addressed: • Treat animal (rat, mice, etc) with drug • Monitor drug duration in animal • Monitor location of drug accumulation • Monitor animal health How do you treat the animal with the drug? How do you monitor the drug concentration in the Animal? How do you determine the drug location? How do you determine the animals health? Tumor size is measured by fluorescence through the mouse skin using quantum dots as a function of drug dosage M. Dahan et al. , Science (2003) 302: 442 -445

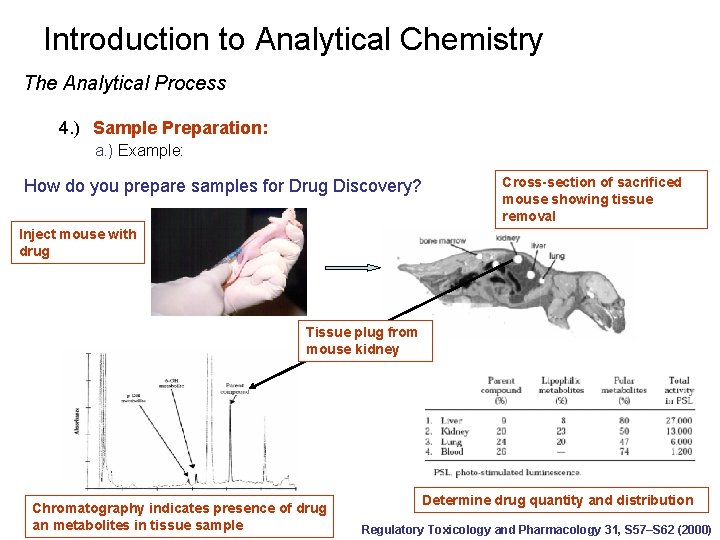
Introduction to Analytical Chemistry The Analytical Process 4. ) Sample Preparation: a. ) Example: How do you prepare samples for Drug Discovery? Cross-section of sacrificed mouse showing tissue removal Inject mouse with drug Tissue plug from mouse kidney Chromatography indicates presence of drug an metabolites in tissue sample Determine drug quantity and distribution Regulatory Toxicology and Pharmacology 31, S 57–S 62 (2000)

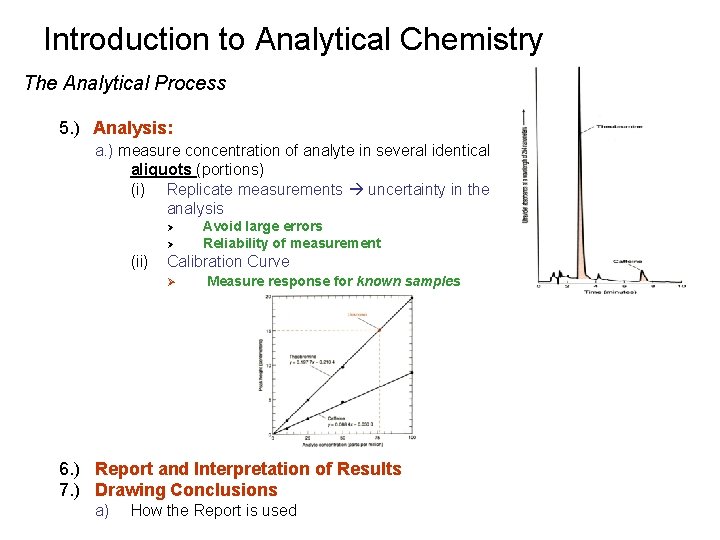
Introduction to Analytical Chemistry The Analytical Process 5. ) Analysis: a. ) measure concentration of analyte in several identical aliquots (portions) (i) Replicate measurements uncertainty in the analysis Ø Ø (ii) Avoid large errors Reliability of measurement Calibration Curve Ø Measure response for known samples 6. ) Report and Interpretation of Results 7. ) Drawing Conclusions a) How the Report is used

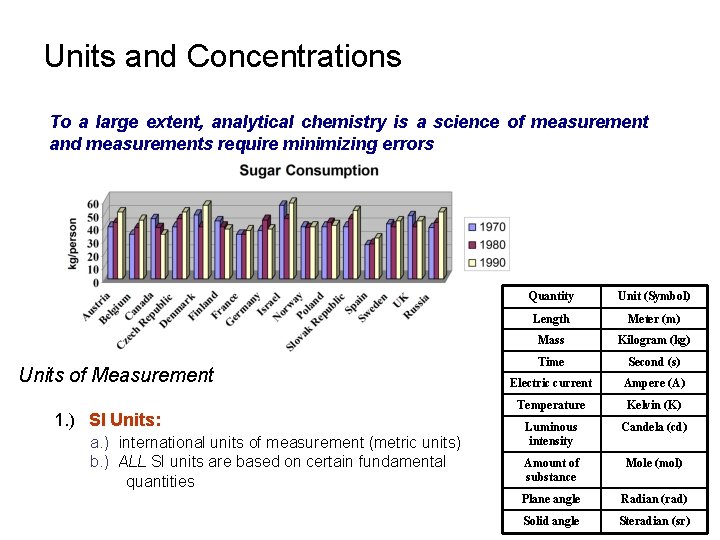
Units and Concentrations To a large extent, analytical chemistry is a science of measurement and measurements require minimizing errors Units of Measurement 1. ) SI Units: a. ) international units of measurement (metric units) b. ) ALL SI units are based on certain fundamental quantities Quantity Unit (Symbol) Length Meter (m) Mass Kilogram (kg) Time Second (s) Electric current Ampere (A) Temperature Kelvin (K) Luminous intensity Candela (cd) Amount of substance Mole (mol) Plane angle Radian (rad) Solid angle Steradian (sr)

Units and Concentrations Units of Measurement Standards of length were once represented by the distance between two marks on a solid metal bar. Copies of these standards were displayed in public places so that people could check the accuracy of the rules they were using. Standards Of Length (1876) Trafalgar Square In 1588, Elizabeth I issued a new standard yard which remained the legal British yard for over 300 years.

Units and Concentrations Units of Measurement History of the meter Origins of the meter go back to at least the 18 th century • Two competing approaches to the definition of a standard unit of length. Ø define the meter as the length of a pendulum having a half-period of one second Ø define the meter as one ten-millionth of the length of the earth's meridian along a quadrant • (1791) French Academy of Sciences chose the meridian Ø force of gravity varies slightly over the surface of the earth, affecting the period of the pendulum. Ø meter equal 10 -7 of the length of the meridian through Paris from pole to the equator. prototype was short by 0. 2 millimeters because researchers miscalculated the flattening of the earth due to its rotation. Ø • (1960) used a definition based upon a wavelength of krypton-86 radiation • (1983) meter replaced by the following definition: Ø The meter is the length of the path traveled by light in vacuum during a time interval of 1/299 792 458 of a second. International Prototype Meter standard bar made of platinum-iridium

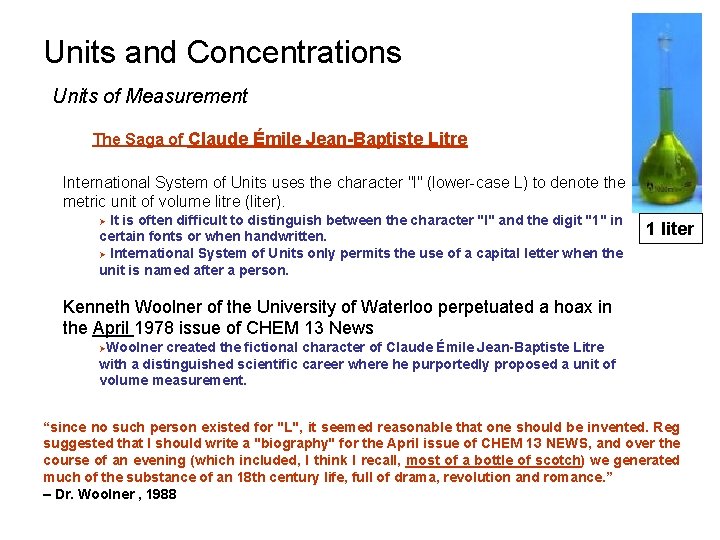
Units and Concentrations Units of Measurement The Saga of Claude Émile Jean-Baptiste Litre International System of Units uses the character "l" (lower-case L) to denote the metric unit of volume litre (liter). It is often difficult to distinguish between the character "l" and the digit "1" in certain fonts or when handwritten. Ø International System of Units only permits the use of a capital letter when the unit is named after a person. Ø 1 liter Kenneth Woolner of the University of Waterloo perpetuated a hoax in the April 1978 issue of CHEM 13 News ØWoolner created the fictional character of Claude Émile Jean-Baptiste Litre with a distinguished scientific career where he purportedly proposed a unit of volume measurement. “since no such person existed for "L", it seemed reasonable that one should be invented. Reg suggested that I should write a "biography" for the April issue of CHEM 13 NEWS, and over the course of an evening (which included, I think I recall, most of a bottle of scotch) we generated much of the substance of an 18 th century life, full of drama, revolution and romance. ” – Dr. Woolner , 1988

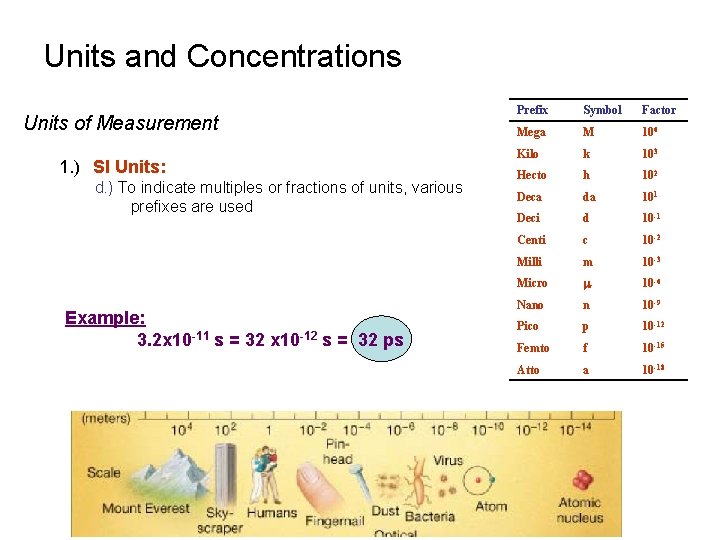
Units and Concentrations Units of Measurement 1. ) SI Units: d. ) To indicate multiples or fractions of units, various prefixes are used Example: 3. 2 x 10 -11 s = 32 x 10 -12 s = 32 ps Prefix Symbol Factor Mega M 106 Kilo k 103 Hecto h 102 Deca da 101 Deci d 10 -1 Centi c 10 -2 Milli m 10 -3 Micro m 10 -6 Nano n 10 -9 Pico p 10 -12 Femto f 10 -15 Atto a 10 -18

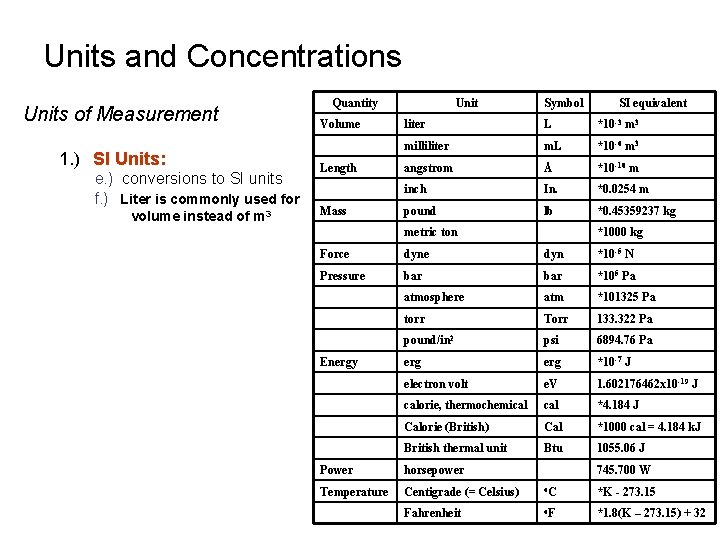
Units and Concentrations Units of Measurement 1. ) SI Units: e. ) conversions to SI units f. ) Liter is commonly used for volume instead of m 3 Quantity Volume Length Mass Unit Symbol SI equivalent liter L *10 -3 m 3 milliliter m. L *10 -6 m 3 angstrom Å *10 -10 m inch In. *0. 0254 m pound lb *0. 45359237 kg metric ton *1000 kg Force dyn *10 -5 N Pressure bar *105 Pa atmosphere atm *101325 Pa torr Torr 133. 322 Pa pound/in 2 psi 6894. 76 Pa erg *10 -7 J electron volt e. V 1. 602176462 x 10 -19 J calorie, thermochemical *4. 184 J Calorie (British) Cal *1000 cal = 4. 184 k. J British thermal unit Btu 1055. 06 J Energy Power horsepower 745. 700 W Temperature Centigrade (= Celsius) o. C *K - 273. 15 Fahrenheit o. F *1. 8(K – 273. 15) + 32

Units and Concentrations Units of Measurement 2. ) Expressions of Concentration: Responsible for what you learned in Chem 113, will use repeatedly in lectures and lab a. ) Molarity (moles/L, or M) b. ) Formality (F) c. ) Percent Composition: (i) (iii) (iv) Weight Percent (wt/wt or w/w) Volume Percent (vol/vol or v/v) Weight-Volume Percent (wt/vol or w/v) parts per thousand (ppt), parts per million (ppm) parts per billion (ppb) 3. ) Solution Preparation: a. ) Dilution of a Solution: Mc. Vc = Md. Vd, where M = Molarity & V = volume

Units and Concentrations Units of Measurement 4. ) Need to be able to answer questions like: a) How many grams of perchloric acid, HCl. O 4, are contained in 37. 6 g of 70. 5 wt% aqueous perchloric acid? How many grams of water are in the same solution? b) What is the maximum volume of 0. 25 M sodium hypochlorite solution(Na. OCl, laundry bleach) that can be prepared by dilution of 1. 00 L of 0. 80 M Na. OCl?