Pressure Image Source MSWord Clipart 4 Main Things











- Slides: 21

Pressure Image Source: MSWord Clipart

4 Main Things You Can Measure About a Gas… Pressure (Pascals) Volume (Liters) Amount (moles) Temperature (Kelvins) Image. Source: MSWord. Clipart

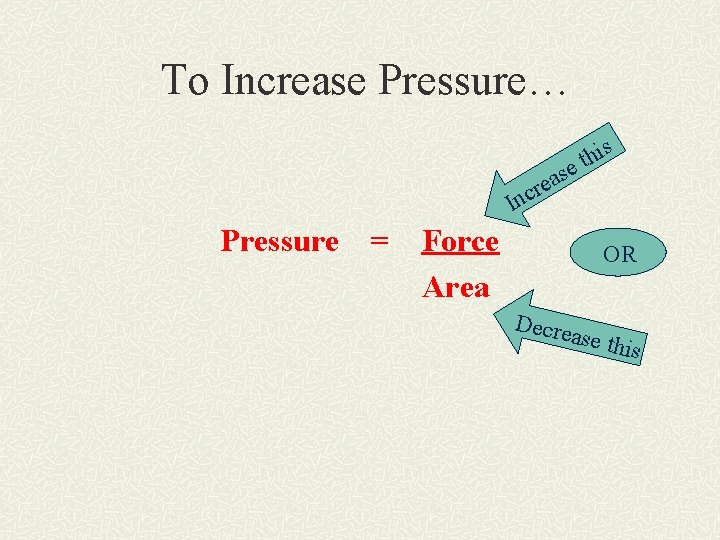
What is Pressure? Pressure = Force Area How would you change the force and/or area to increase the pressure?

To Increase Pressure… s i h et s a e cr In Pressure = Force Area OR Decre ase th is

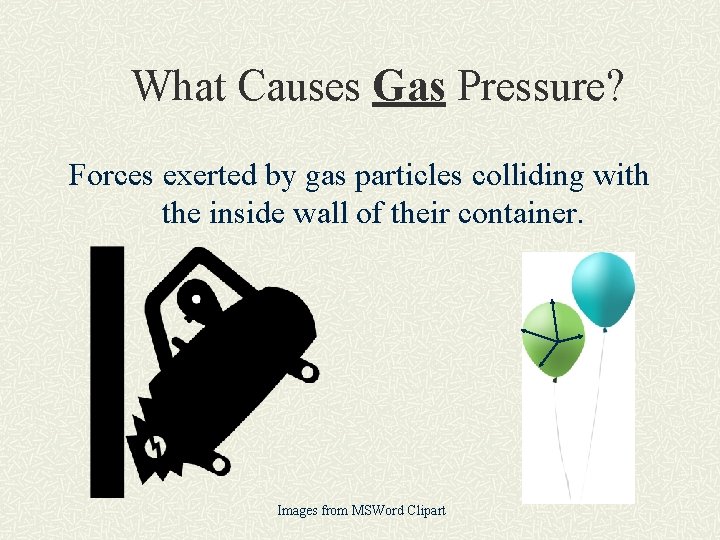
What Causes Gas Pressure? Forces exerted by gas particles colliding with the inside wall of their container. Images from MSWord Clipart

Is the Pressure High or Low in Outer Space? Image from MSWord Clipart

Answer… Low (or zero); there are few gas particles in outer space (it’s a vacuum), so there isn’t anything available to have collisions and exert forces on objects

Atmospheric Pressure The pressure the Earth’s atmosphere exerts on objects due to the weight of the air pushing down on it. Image from MSWord Clipart

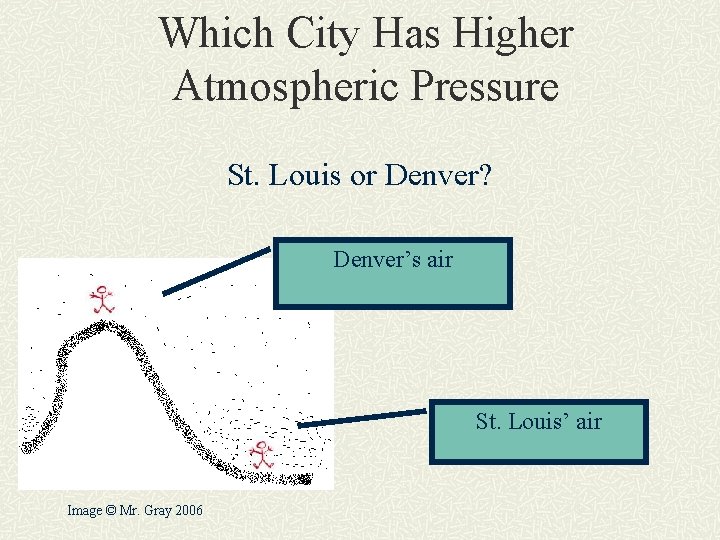
Which City Has Higher Atmospheric Pressure St. Louis or Denver? Denver’s air St. Louis’ air Image © Mr. Gray 2006

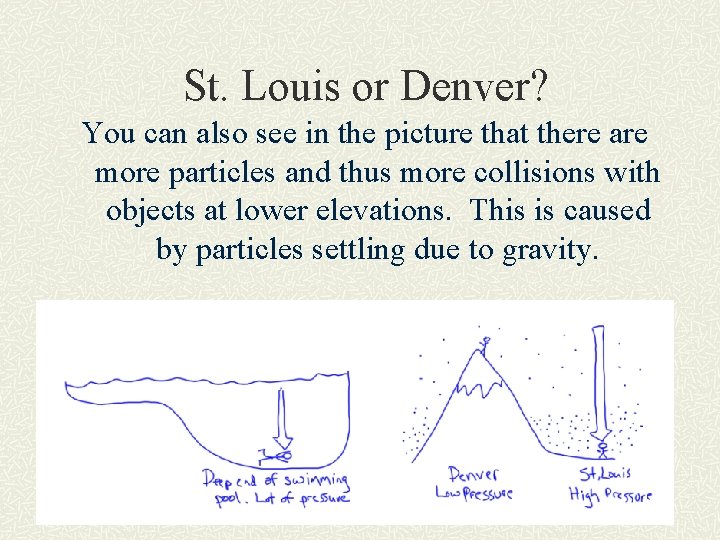
St. Louis or Denver? St. Louis has higher atmospheric pressure. It is like being in the “deep end” of the atmosphere. There is slightly more air above you so it weighs more and pushes down on you harder.

St. Louis or Denver? You can also see in the picture that there are more particles and thus more collisions with objects at lower elevations. This is caused by particles settling due to gravity.

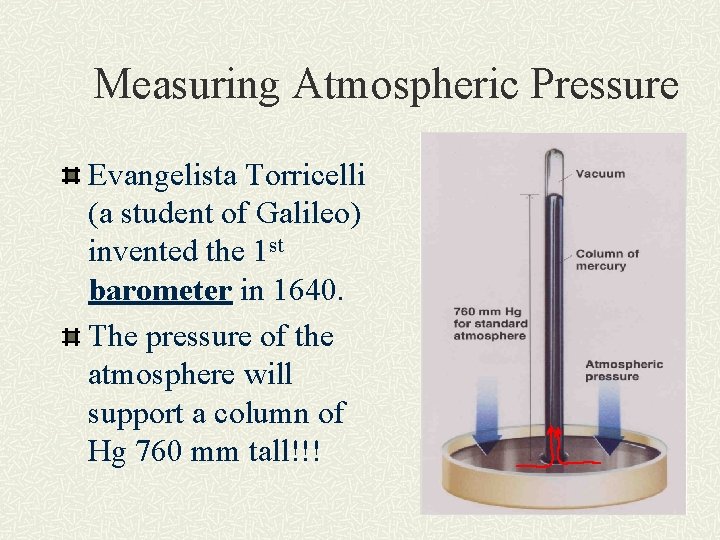
Measuring Atmospheric Pressure Evangelista Torricelli (a student of Galileo) invented the 1 st barometer in 1640. The pressure of the atmosphere will support a column of Hg 760 mm tall!!!

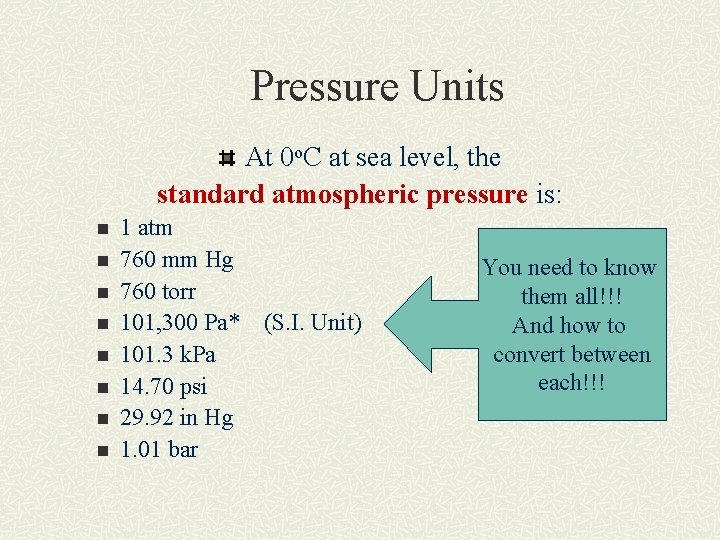
Pressure Units At 0 o. C at sea level, the standard atmospheric pressure is: n n n n 1 atm 760 mm Hg 760 torr 101, 300 Pa* 101. 3 k. Pa 14. 70 psi 29. 92 in Hg 1. 01 bar (S. I. Unit) You need to know them all!!! And how to convert between each!!!

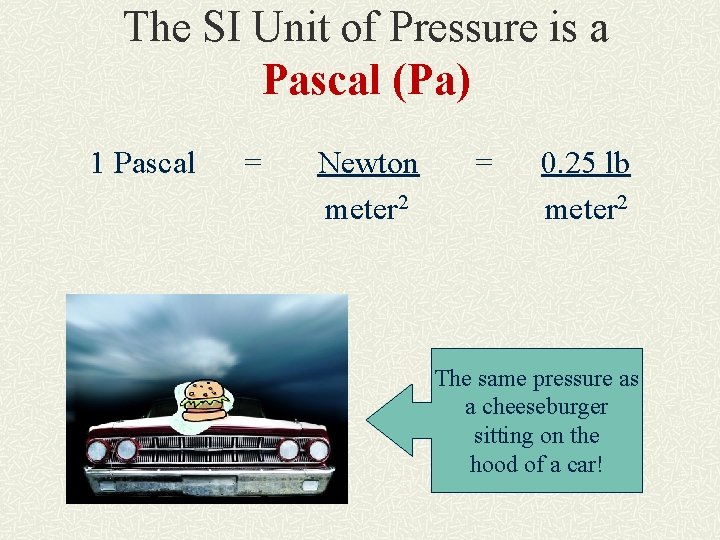
The SI Unit of Pressure is a Pascal (Pa) 1 Pascal = Newton meter 2 = 0. 25 lb meter 2 The same pressure as a cheeseburger sitting on the hood of a car!

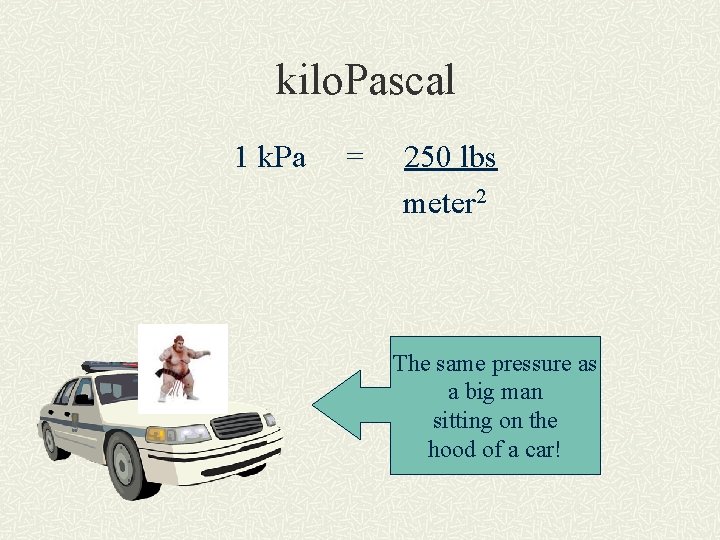
kilo. Pascal 1 k. Pa = 250 lbs meter 2 The same pressure as a big man sitting on the hood of a car!

1 atm is equal to… 1. 670 mm Hg 2. 14. 70 barr 3. 29. 92 mm Hg 4. 760 k. Pa 5. 101. 3 k. Pa

1 atm is equal to… 1. 670 mm Hg 2. 14. 70 barr 3. 29. 92 mm Hg 4. 760 k. Pa 5. 101. 3 k. Pa

Pressure Unit Conversions A balloon has a pressure of 125 k. Pa. How many mm. Hg is that? 125 k. Pa x 760 mm. Hg 101. 3 k. Pa I made this conversion Factor from the units I memorized! = 938 mm. Hg

Is Atmospheric Pressure High? Demos of atmospheric pressure Soda can n Flask of water and card n Balloon on flask n Calculate pressure on cylindrical person n n 2 rh n + 2( r 2) Put student in a vacuum

Why doesn’t atmospheric pressure crush us? 1. Because the pressure inside us balances it 2. Because atmospheric pressure is low 3. Because the pressure inside us is greater

Why doesn’t atmospheric pressure crush us? 1. Because the pressure inside us balances it 2. Because atmospheric pressure is low 3. Because the pressure inside us is greater