Predictors of glycemic control with the clinical use











- Slides: 22

Predictors of glycemic control with the clinical use of the Mini. Med™ 670 G hybrid closed-loop insulin delivery system in children, adolescents, and young adults with type 1 diabetes. Maggie Daseler PGY-1 Pharmacy Resident Children’s Minnesota- St. Paul

Learning Objectives 1. Understand the mechanisms of the Mini. Med 670 G pump including the Auto Mode and suspend before low function. 2. Discuss the efficacy and safety of Mini. Med 670 G for tight glucose control. 3. Assess the long-term adherence of the Mini. Med 670 G insulin pump. © 2019 2

Disclosures © 2019 • Children's makes no representations or warranties about the accuracy, reliability, or completeness of the content. Content is provided "as is" and is for informational use only. It is not a substitute for professional medical advice, diagnosis, or treatment. Children’s disclaims all warranties, express or implied, statutory or otherwise, including without limitation the implied warranties of merchantability, non-infringement of third parties’ rights, and fitness for a particular purpose. • This content was developed for use in Children’s patient care environment and may not be suitable for use in other patient care environments. Children’s does not endorse, certify, or assess third parties’ competency. You hold all responsibility for your use or nonuse of the content. Children’s shall not be liable for claims, losses, or damages arising from or related to any use or misuse of the content. • This content and its related discussions are privileged and confidential under Minnesota’s peer review statute (Minn. Stat. § 145. 61 et. seq. ). Do not disclose unless appropriately authorized. Notwithstanding the foregoing, content may be subject to copyright or trademark law; use of such information requires Children’s permission. • This content may include patient protected health information. You agree to comply with all applicable state and federal laws protecting patient privacy and security including the Minnesota Health Records Act and the Health Insurance Portability and Accountability Act and its implementing regulations as amended from time to time. • Please ask if you have any questions about these disclaimers and/or confidentiality protections. 3

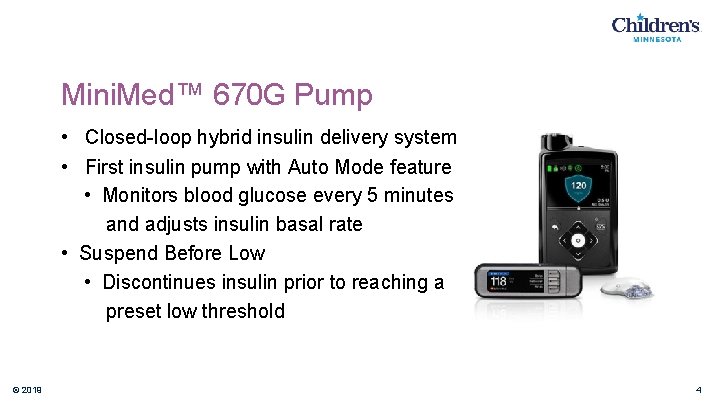
Mini. Med™ 670 G Pump • Closed-loop hybrid insulin delivery system • First insulin pump with Auto Mode feature • Monitors blood glucose every 5 minutes and adjusts insulin basal rate • Suspend Before Low • Discontinues insulin prior to reaching a preset low threshold © 2019 4

Methods • Retrospective Chart Review • Performed at Children’s Minnesota Mc. Neely Pediatric Diabetes Center • Extension Study • Data was collected up to 96 weeks after initiation of the Mini. Med™ 670 G system © 2019 5

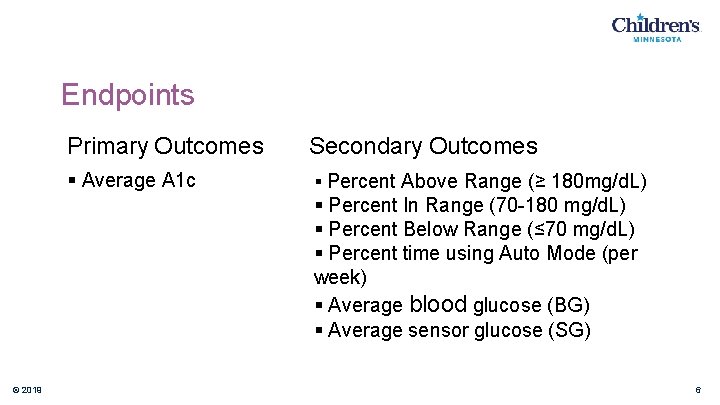
Endpoints Primary Outcomes Secondary Outcomes § Average A 1 c § Percent Above Range (≥ 180 mg/d. L) § Percent In Range (70 -180 mg/d. L) § Percent Below Range (≤ 70 mg/d. L) § Percent time using Auto Mode (per week) § Average blood glucose (BG) § Average sensor glucose (SG) © 2019 6

Criteria • Inclusion Criteria • Patients initiating insulin pump therapy with the Mini. Med 670 G between 3/1/17 – 11/10/18 • Patient is aged 4 -25 years old at time of 670 G initiation • Patient is clinically diagnosed with Type 1 Diabetes Mellitus • Patient had a clinic visit and seen in person by a provider in Mc. Neely Diabetes Center • Exclusion Criteria • Patient is clinically diagnosed with Type 2 Diabetes Mellitus • Patient declined research participation initial intake form © 2019 7

Preliminary Results

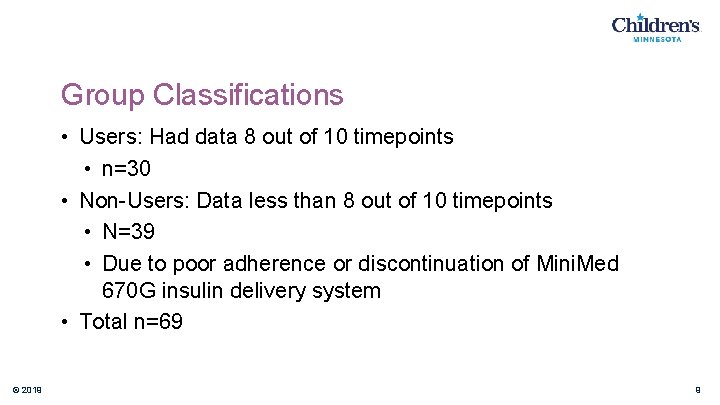
Group Classifications • Users: Had data 8 out of 10 timepoints • n=30 • Non-Users: Data less than 8 out of 10 timepoints • N=39 • Due to poor adherence or discontinuation of Mini. Med 670 G insulin delivery system • Total n=69 © 2019 9

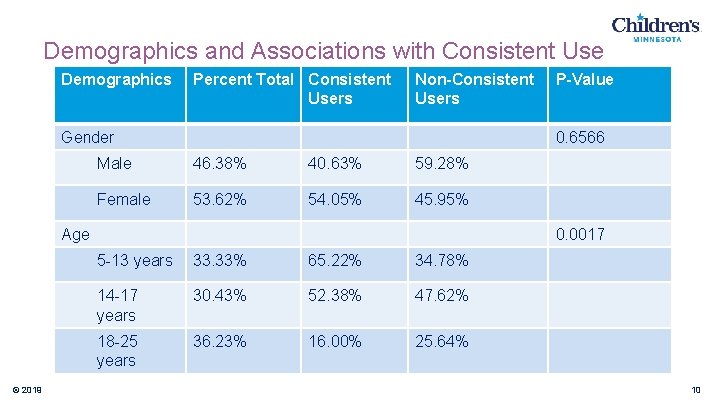
Demographics and Associations with Consistent Use Demographics Percent Total Consistent Users Gender Non-Consistent Users 0. 6566 Male 46. 38% 40. 63% 59. 28% Female 53. 62% 54. 05% 45. 95% Age © 2019 P-Value 0. 0017 5 -13 years 33. 33% 65. 22% 34. 78% 14 -17 years 30. 43% 52. 38% 47. 62% 18 -25 years 36. 23% 16. 00% 25. 64% 10

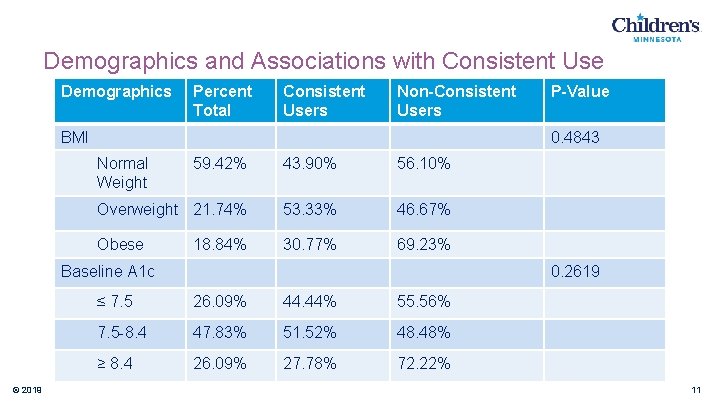
Demographics and Associations with Consistent Use Demographics Percent Total Consistent Users Non-Consistent Users BMI 0. 4843 Normal Weight 59. 42% 43. 90% 56. 10% Overweight 21. 74% 53. 33% 46. 67% Obese 30. 77% 69. 23% 18. 84% Baseline A 1 c © 2019 P-Value 0. 2619 ≤ 7. 5 26. 09% 44. 44% 55. 56% 7. 5 -8. 4 47. 83% 51. 52% 48. 48% ≥ 8. 4 26. 09% 27. 78% 72. 22% 11

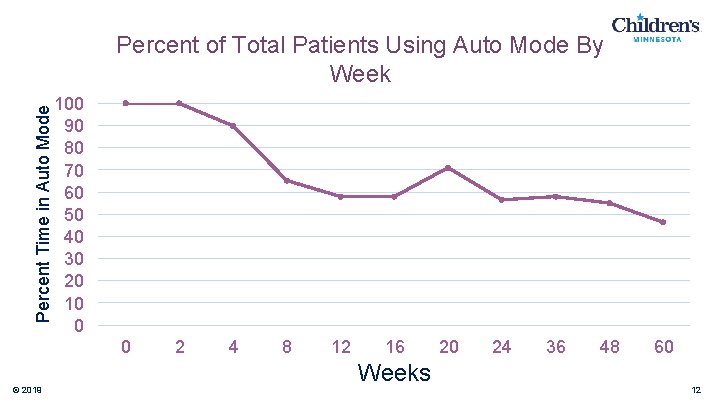
Percent Time in Auto Mode Percent of Total Patients Using Auto Mode By Week 100 90 80 70 60 50 40 30 20 10 0 0 © 2019 2 4 8 12 16 Weeks 20 24 36 48 60 12

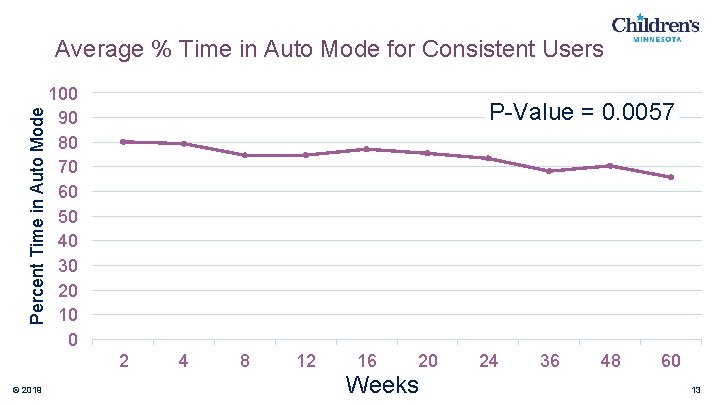
Percent Time in Auto Mode Average % Time in Auto Mode for Consistent Users 100 90 80 70 60 50 40 30 20 10 0 P-Value = 0. 0057 2 © 2019 4 8 12 16 20 Weeks 24 36 48 60 13

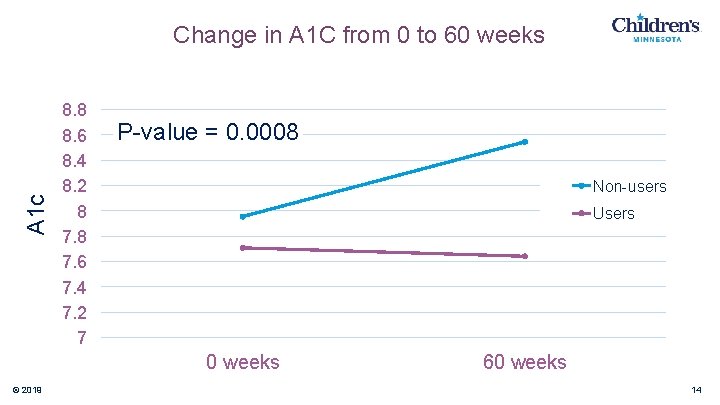
A 1 c Change in A 1 C from 0 to 60 weeks 8. 8 8. 6 8. 4 8. 2 8 7. 6 7. 4 7. 2 7 P-value = 0. 0008 Non-users Users 0 weeks © 2019 60 weeks 14

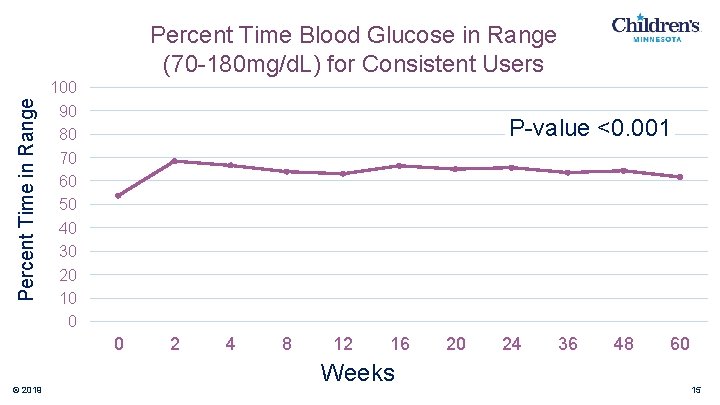
Percent Time in Range Percent Time Blood Glucose in Range (70 -180 mg/d. L) for Consistent Users 100 90 80 70 60 50 40 30 20 10 0 P-value <0. 001 0 © 2019 2 4 8 12 16 Weeks 20 24 36 48 60 15

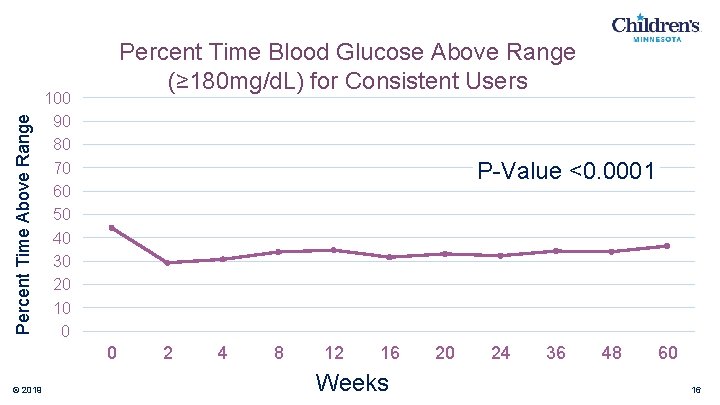
Percent Time Above Range Percent Time Blood Glucose Above Range (≥ 180 mg/d. L) for Consistent Users 100 90 80 70 60 50 40 30 20 10 0 P-Value <0. 0001 0 © 2019 2 4 8 12 16 Weeks 20 24 36 48 60 16

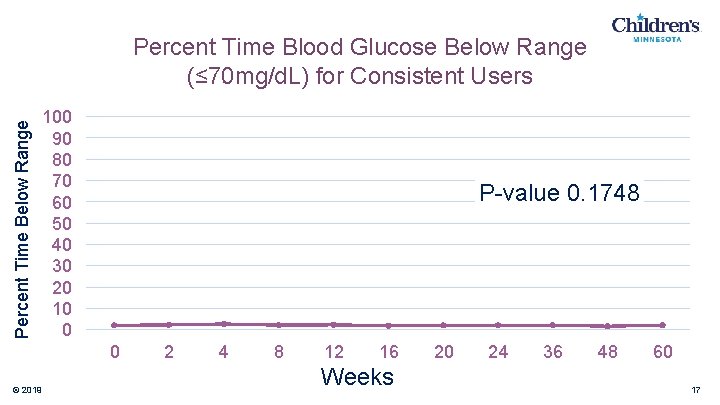
Percent Time Below Range Percent Time Blood Glucose Below Range (≤ 70 mg/d. L) for Consistent Users 100 90 80 70 60 50 40 30 20 10 0 P-value 0. 1748 0 © 2019 2 4 8 12 16 Weeks 20 24 36 48 60 17

Conclusions • Consistent use of Mini. Med™ 670 G decreased A 1 c • Consistent use of Mini. Med™ 670 G increased % time in range • These were statistically significant findings • Data will continue to be collected analyzed up to 96 weeks from baseline © 2019 18

Acknowledgements • • © 2019 Laura Gandrud, MD Carlo Henson, Pharm. D Amanda Nickel David Watson 19

Questions © 2019 20

References 1. 2. 3. 4. © 2019 Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicenter, 12 -week randomized trial. Lancet 2018; 392: 1321 -29. De Bock M, Mc. Auley SA, Abraham MB, et al. Effect of 6 months hybrid closedloop insulin delivery in young people with type 1 diabetes: a randomized controlled trial protocol. BMJ Open 2018; 8: 1 -8. Aleppo G, Webb KM. Integrated insulin pump and continuous glucose monitoring technology in diabetes care today: a perspective of real-life experience with the Minimed™ 670 G hybrid closed-loop system. Endocrin Practice. July 2018; 24(7): 684 -692. Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabetes UK. September 2018; 1 -8. 21

 Roc curve with multiple predictors
Roc curve with multiple predictors Glycemic control algorithm
Glycemic control algorithm Glycemic index of oats
Glycemic index of oats Glycemic index of tofu
Glycemic index of tofu Gi of cocoyam
Gi of cocoyam Mappillai samba rice glycemic index
Mappillai samba rice glycemic index Symptoms of diabettes
Symptoms of diabettes Lactose glycemic index
Lactose glycemic index Oreo high fructose corn syrup
Oreo high fructose corn syrup Low glycemic index foods india
Low glycemic index foods india Low glycemic index foods list pdf india
Low glycemic index foods list pdf india Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ










































