Power Point to Accompany Principles and Applications of








- Slides: 25

Power Point to Accompany Principles and Applications of Inorganic, Organic, and Biological Chemistry Denniston, Topping, and Caret 4 th ed Chapter 14 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 1

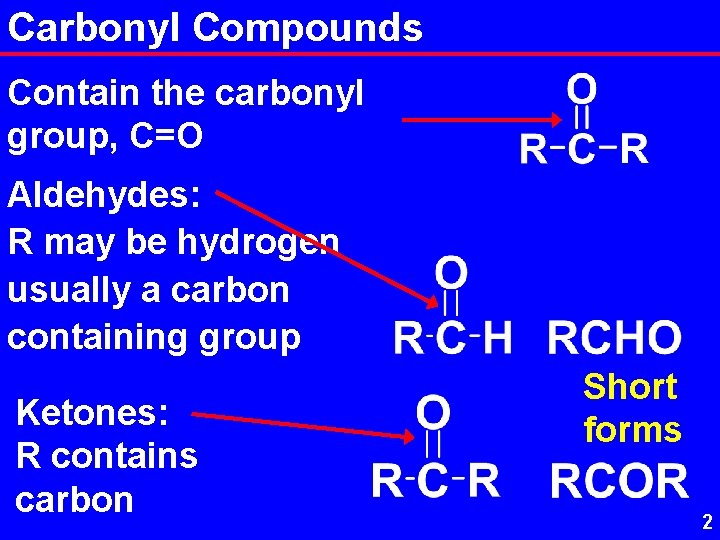
Carbonyl Compounds Contain the carbonyl group, C=O Aldehydes: R may be hydrogen usually a carbon containing group Ketones: R contains carbon Short forms 2

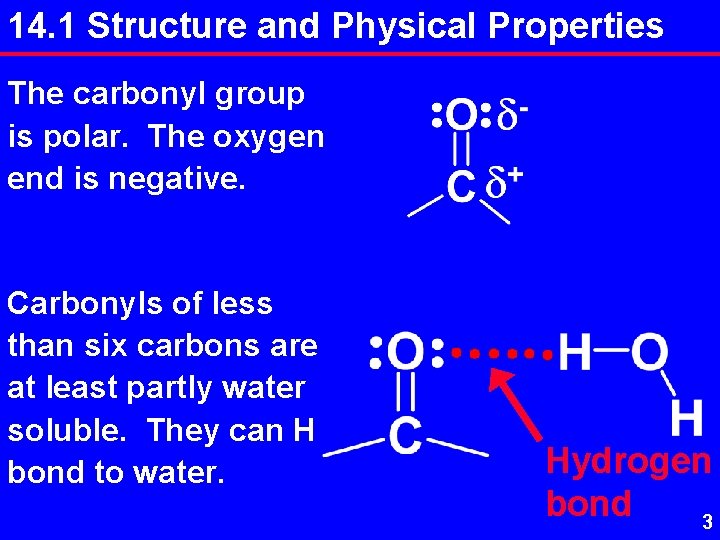
14. 1 Structure and Physical Properties The carbonyl group is polar. The oxygen end is negative. Carbonyls of less than six carbons are at least partly water soluble. They can H bond to water. Hydrogen bond 3

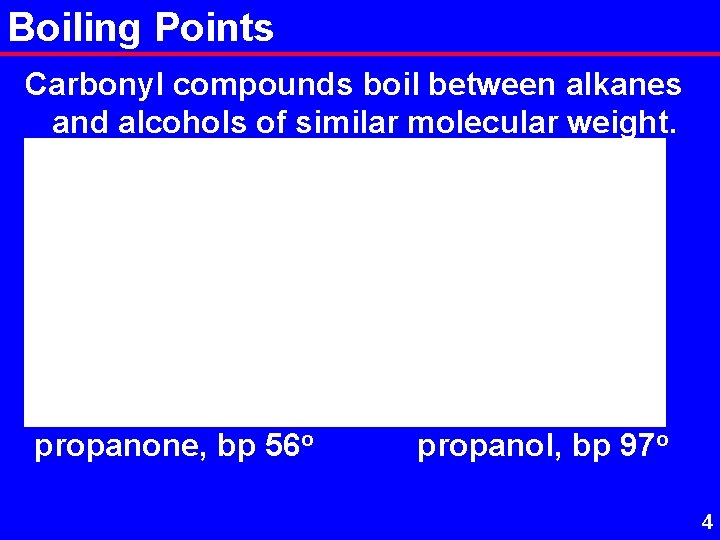
Boiling Points Carbonyl compounds boil between alkanes and alcohols of similar molecular weight. butane, bp -43 o propanone, bp 56 o propanal, bp 50 o propanol, bp 97 o 4

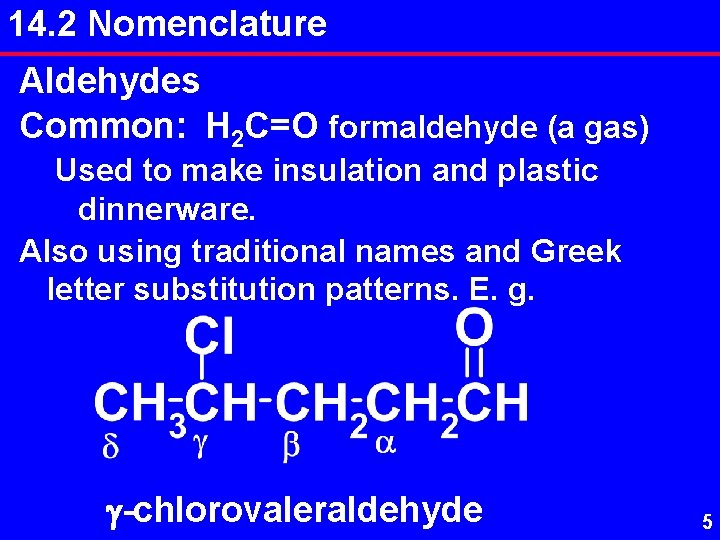
14. 2 Nomenclature Aldehydes Common: H 2 C=O formaldehyde (a gas) Used to make insulation and plastic dinnerware. Also using traditional names and Greek letter substitution patterns. E. g. g-chlorovaleraldehyde 5

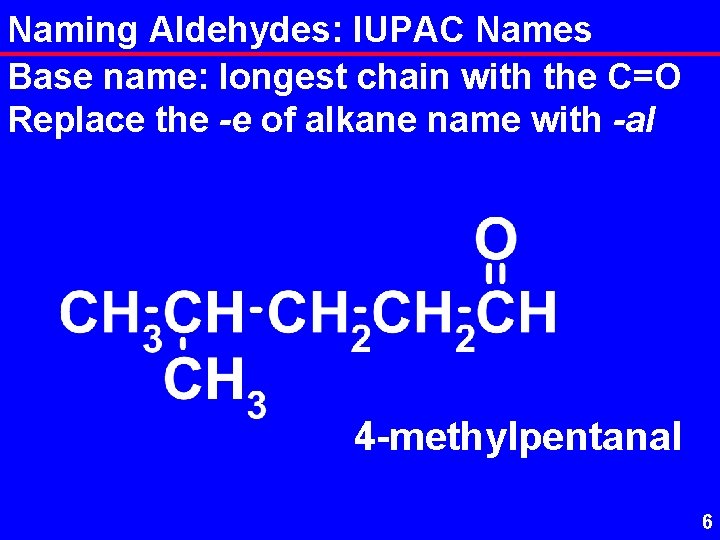
Naming Aldehydes: IUPAC Names Base name: longest chain with the C=O Replace the -e of alkane name with -al 4 -methylpentanal 6

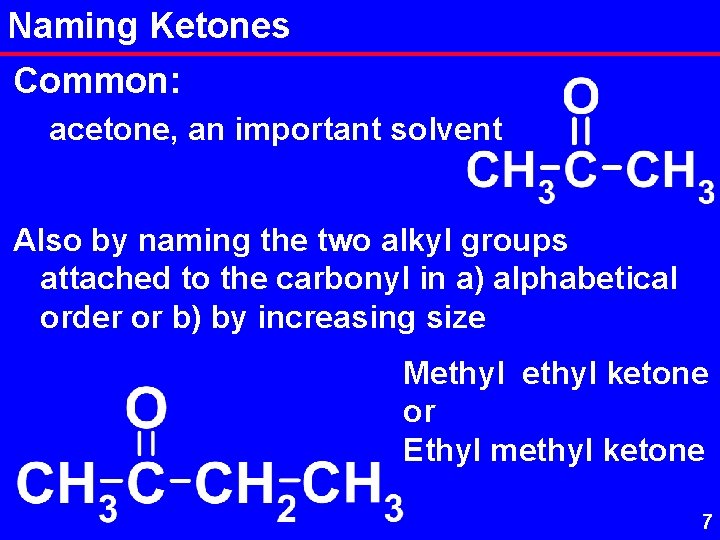
Naming Ketones Common: acetone, an important solvent Also by naming the two alkyl groups attached to the carbonyl in a) alphabetical order or b) by increasing size Methyl ketone or Ethyl methyl ketone 7

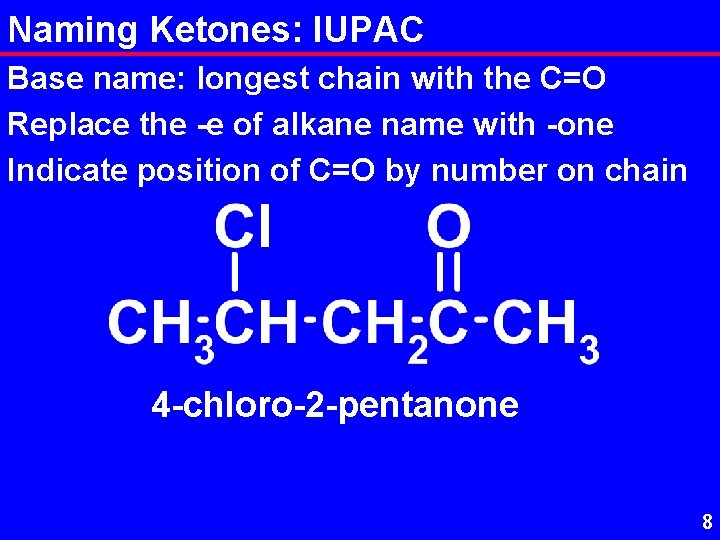
Naming Ketones: IUPAC Base name: longest chain with the C=O Replace the -e of alkane name with -one Indicate position of C=O by number on chain 4 -chloro-2 -pentanone 8

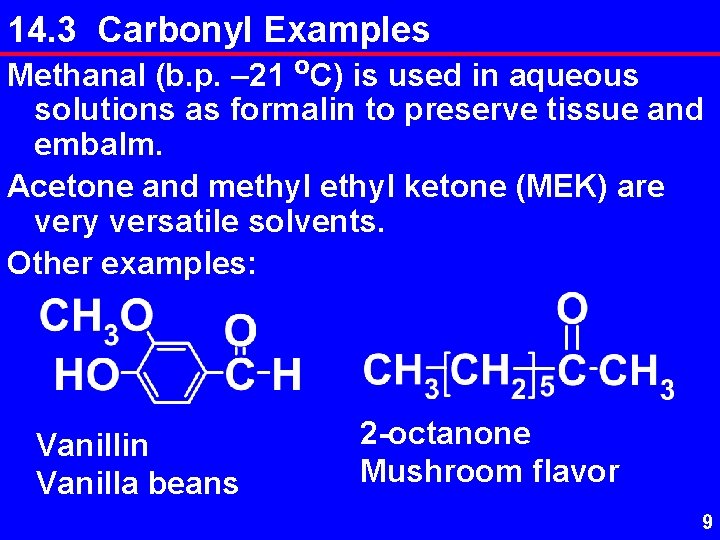
14. 3 Carbonyl Examples Methanal (b. p. – 21 o. C) is used in aqueous solutions as formalin to preserve tissue and embalm. Acetone and methyl ketone (MEK) are very versatile solvents. Other examples: Vanillin Vanilla beans 2 -octanone Mushroom flavor 9

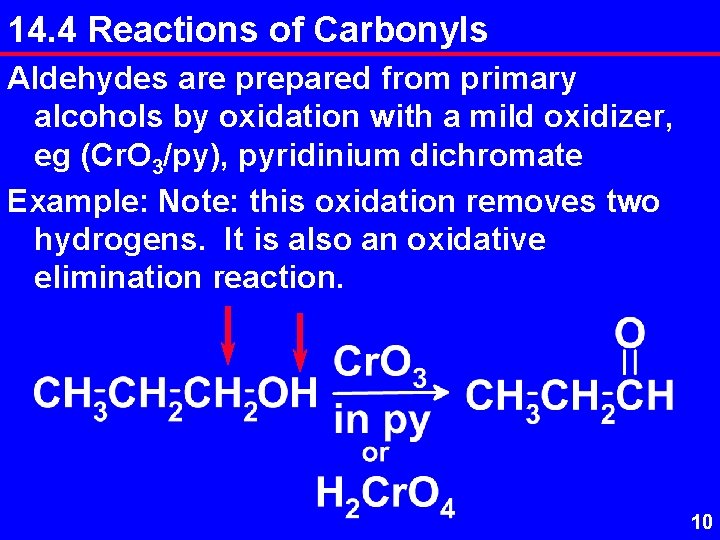
14. 4 Reactions of Carbonyls Aldehydes are prepared from primary alcohols by oxidation with a mild oxidizer, eg (Cr. O 3/py), pyridinium dichromate Example: Note: this oxidation removes two hydrogens. It is also an oxidative elimination reaction. 10

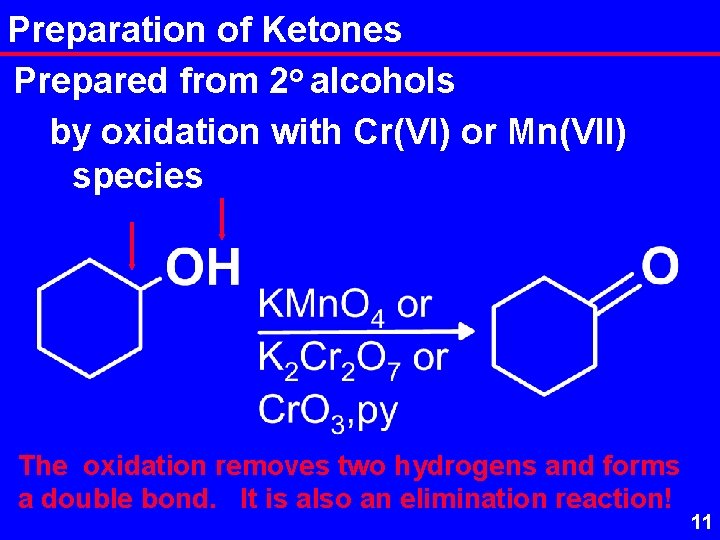
Preparation of Ketones Prepared from 2 o alcohols by oxidation with Cr(VI) or Mn(VII) species The oxidation removes two hydrogens and forms a double bond. It is also an elimination reaction! 11

Reactions of Carbonyls 1. Redox a) Aldehydes: oxidized to carboxylic acids b) Aldehydes and ketones are reduced to alcohols: aldehydes to primary alcohols and ketones to secondary alcohols 2. Addition a) of hydrogen to give alcohols b) of alcohols to give hemiacetals, hemiketals, and ketals c) of aldehydes/ketones to give aldol (bhydroxy carbonyl) products 12

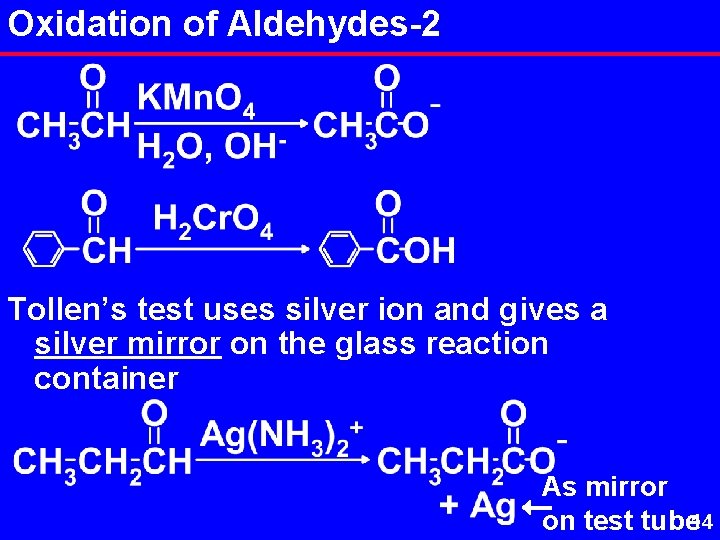
Oxidation of Aldehydes are easily oxidized to carboxylic acids by almost any oxidizing agent. Visual tests for the aldehyde functional group based on its easy oxidation are: Tollen’s Test Silver ion is the oxidizer, as Ag(NH 3)2 + Benedict’s Test Cu 2+ in a basic solution is the oxidizer. Solution is a distinctive blue color. Color fades with rxn. 13

Oxidation of Aldehydes-2 Tollen’s test uses silver ion and gives a silver mirror on the glass reaction container As mirror on test tube 14

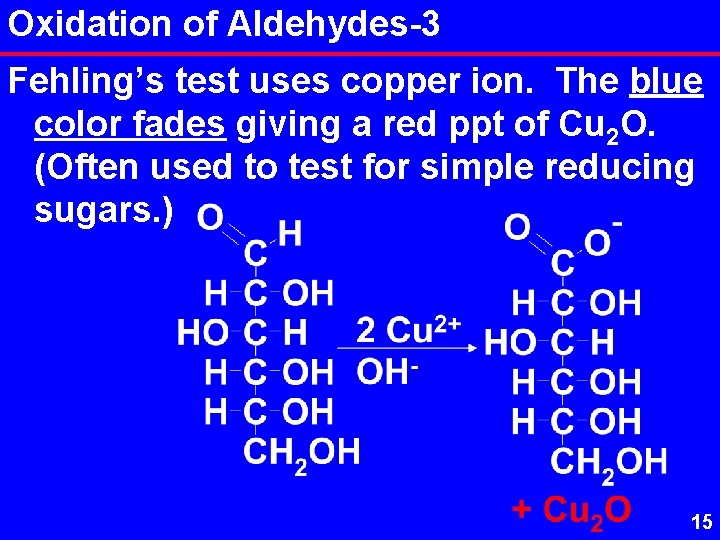
Oxidation of Aldehydes-3 Fehling’s test uses copper ion. The blue color fades giving a red ppt of Cu 2 O. (Often used to test for simple reducing sugars. ) 15

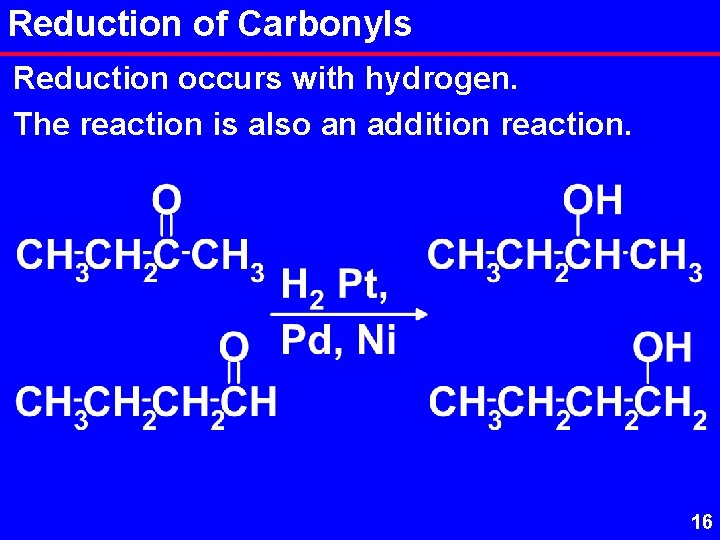
Reduction of Carbonyls Reduction occurs with hydrogen. The reaction is also an addition reaction. 16

Additions to Carbonyls: Alcohols Carbonyls add one molecule of alcohol to give usually unstable hemiacetals and hemiketals Hemiacetal(ketal) carbons are part of both alcohol and ether functions and are a new functional group 17

Additions to Carbonyls: Alcohols-2 Acid catalyzes both aldehydes and ketones to react with two molecules of alcohol to give acetals and ketals Acetal(ketal) carbons are part of two ether groups and are a new functional group 18

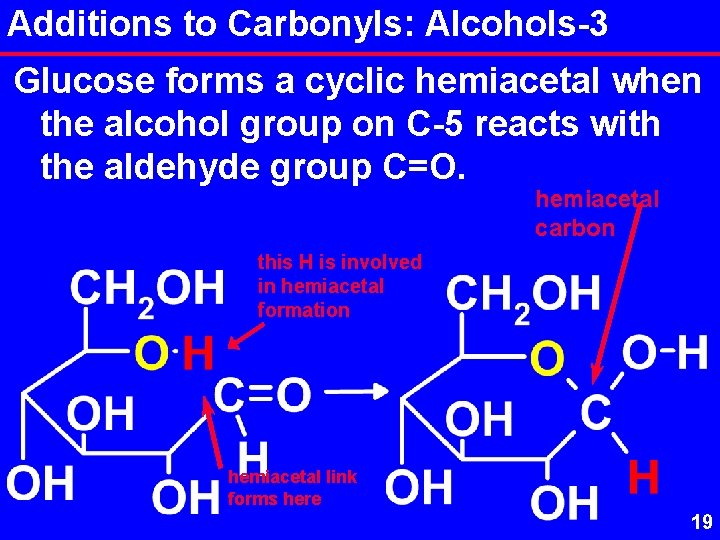
Additions to Carbonyls: Alcohols-3 Glucose forms a cyclic hemiacetal when the alcohol group on C-5 reacts with the aldehyde group C=O. hemiacetal carbon this H is involved in hemiacetal formation hemiacetal link forms here 19

Hydrolysis of (Hemi)acetals/ketals Both hemiacetals and acetals (and ketone analogs) hydrolyze (react with water). An acid catalyst takes them back to the carbonyl compound and the alcohol(s). acetal hemiketal 20

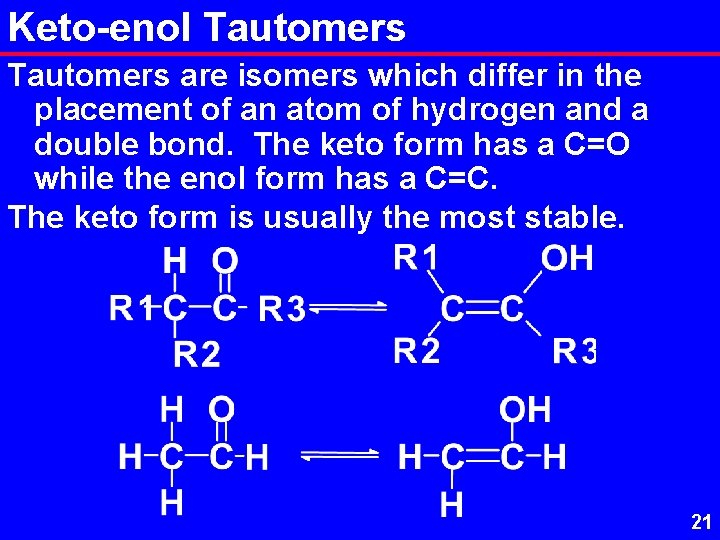
Keto-enol Tautomers are isomers which differ in the placement of an atom of hydrogen and a double bond. The keto form has a C=O while the enol form has a C=C. The keto form is usually the most stable. 21

Additions to Carbonyls: Aldol Condensation Self Addition or Condensation Uses two molecules of the same aldehyde or ketone. The alpha carbon of the second molecule adds to the carbonyl carbon of the first molecule. Strong base such as hydroxide catalyzes the reaction. 22

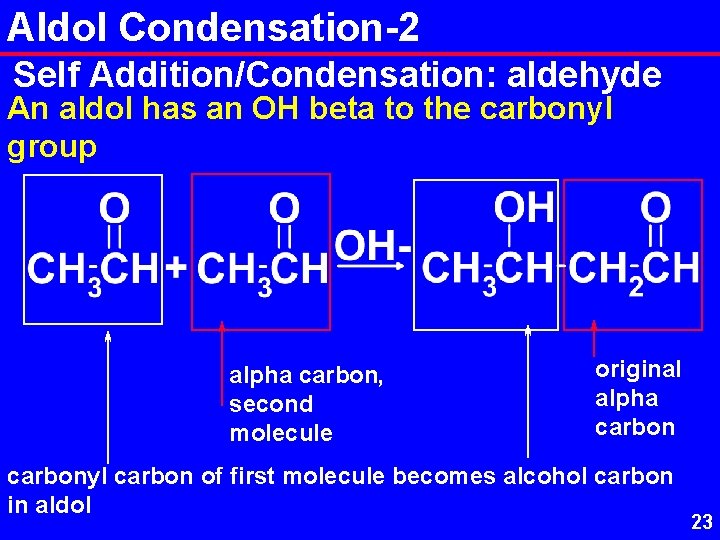
Aldol Condensation-2 Self Addition/Condensation: aldehyde An aldol has an OH beta to the carbonyl group alpha carbon, second molecule original alpha carbonyl carbon of first molecule becomes alcohol carbon in aldol 23

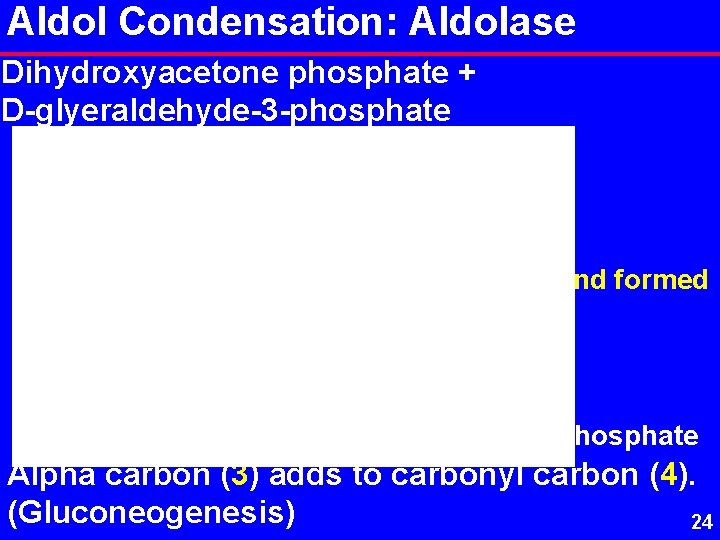
Aldol Condensation: Aldolase Dihydroxyacetone phosphate + D-glyeraldehyde-3 -phosphate Bond formed D-fructose-1, 6 -bisphosphate Alpha carbon (3) adds to carbonyl carbon (4). (Gluconeogenesis) 24

The End Carbonyl Compounds 25
 Hebrews 6:9-12 sermon
Hebrews 6:9-12 sermon Accompany chapter 1
Accompany chapter 1 Superficial veins of upper limb
Superficial veins of upper limb Inkjet printers are considered legacy technology
Inkjet printers are considered legacy technology Ac power formula
Ac power formula Powerbi in powerpoint
Powerbi in powerpoint Point point power
Point point power Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Pearson engineering
Pearson engineering Learning principles and applications
Learning principles and applications Power electronics circuits devices and applications
Power electronics circuits devices and applications Principles of network applications in computer networks
Principles of network applications in computer networks Principles of network applications
Principles of network applications Maximum power transfer calculator
Maximum power transfer calculator Hub-and-spoke system pros and cons
Hub-and-spoke system pros and cons Solar power satellites and microwave power transmission
Solar power satellites and microwave power transmission Actual power
Actual power Dispersive power is directly proportional to
Dispersive power is directly proportional to 3 realms of heaven
3 realms of heaven What is the disadvantage of microsoft powerpoint
What is the disadvantage of microsoft powerpoint Block organization vs point by point
Block organization vs point by point Bubble point test formula
Bubble point test formula Point of difference and point of parity
Point of difference and point of parity Ideas for one point perspective drawing
Ideas for one point perspective drawing