Polyacrylamide Gel Electrophoresis PAGE of Proteins Ph D








- Slides: 18


Polyacrylamide Gel Electrophoresis (PAGE) of Proteins • Ph. D. Dhurata Feta • Ph. D. Artiona Laze ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

NETCHEM Remote Access Laboratory Guide Evaluation of wheat genotypes by polyacrilamide gel electrophoresis In this laboratory work, you will: • • • Understand the principle of SDS-PAGE To become familiar with SDS-PAGE setup Determine of protein molecular weight ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

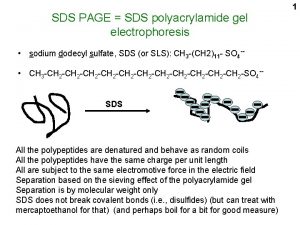
Background Wheat is one of the most important cereal crops worldwide in terms of production and utilization. The ability of wheat flour to be processed into different food is largely determined by the proteins. Mature wheat grain contain 8% to 20% protein. Wheat is unique among the edible grain because wheat flour has the protein complex called “gluten”. The gluten proteins consist of monomeric gliadins and polymeric glutenins. Glutenins and gliadins are recognized as the major wheat storage proteins. Glutenins can be broadly classified into two groups, the high molecular weigh (HMW) and low molecular weigh (LMW) subunits, with molecular weigh (Mw) range of 100 to 140 KDa and 30 to 55 KDa respectively, according to mobility on SDS-PAGE. Sodium Dodecyl Sulfate-Polyacrylamide gel Electrophoresis (SDS-PAGE), is a method where charged molecules in solution, mainly proteins, migrate in response to an electrical field. -This method separates proteins based primarily on their molecular weights. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Aparatus • • • Centrifuge and centrifuge tubes. Analytical balance - capable of accurate weighing's to 0. 01 g. Heating source (e. g. , hot plate) Vortex (Mixer) mortar and pestle mill Gel casting apparatus Graduated cylinder or equivalent volume measuring device. Volumetric Flasks – 50 m. L, 100 m. L. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Procedure: Protein Extraction • • Extraction buffer (SDS-based extraction) – TRIS 1 M, p. H 6. 8 – Water – SDS – 2 -mercaptoethanol/heat – Bromphenol blue 1%. Centrifuge extract ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

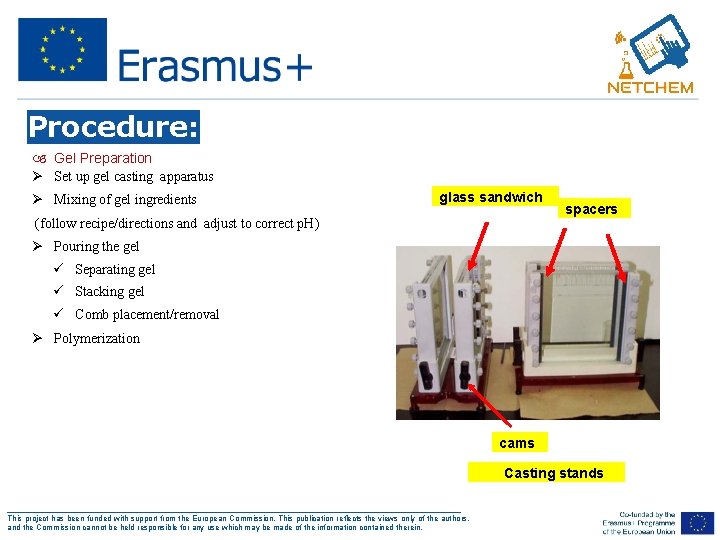
Procedure: Gel Preparation Ø Set up gel casting apparatus Ø Mixing of gel ingredients glass sandwich (follow recipe/directions and adjust to correct p. H) spacers Ø Pouring the gel ü Separating gel ü Stacking gel ü Comb placement/removal Ø Polymerization cams Casting stands ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

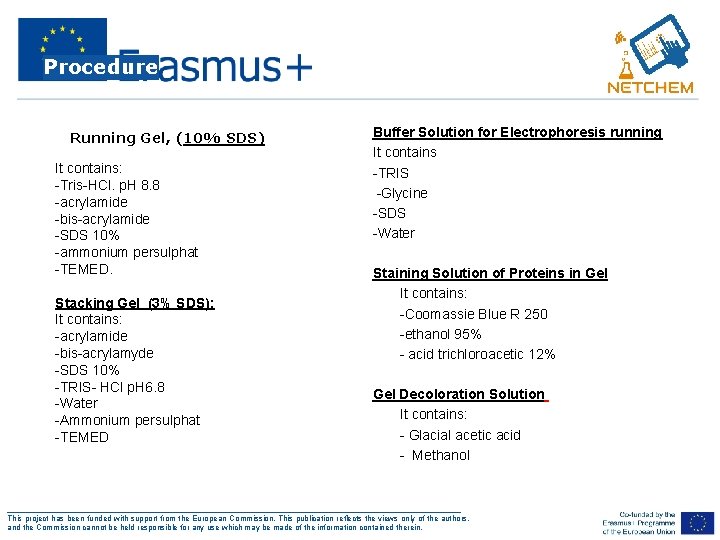
Procedure • Running Gel, (10% SDS) It contains: -Tris-HCl. p. H 8. 8 -acrylamide -bis-acrylamide -SDS 10% -ammonium persulphat -TEMED. Stacking Gel (3% SDS): It contains: -acrylamide -bis-acrylamyde -SDS 10% -TRIS- HCl p. H 6. 8 -Water -Ammonium persulphat -TEMED Buffer Solution for Electrophoresis running It contains -TRIS -Glycine -SDS -Water Staining Solution of Proteins in Gel • It contains: • -Coomassie Blue R 250 • -ethanol 95% • - acid trichloroacetic 12% Gel Decoloration Solution • It contains: • - Glacial acetic acid • - Methanol ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Ø The purpose of the stacking gel is to condense the protein into a tight band before running it through the separating gel. This allows better resolution in the separating gel. Ø The stacking effect works because the stacking gel is made up of a lower concentration of polyacrylamide than the separating gel. Ø The protein stacks because the proteins move very quickly through the stacking gel, only to run into the separating gel ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

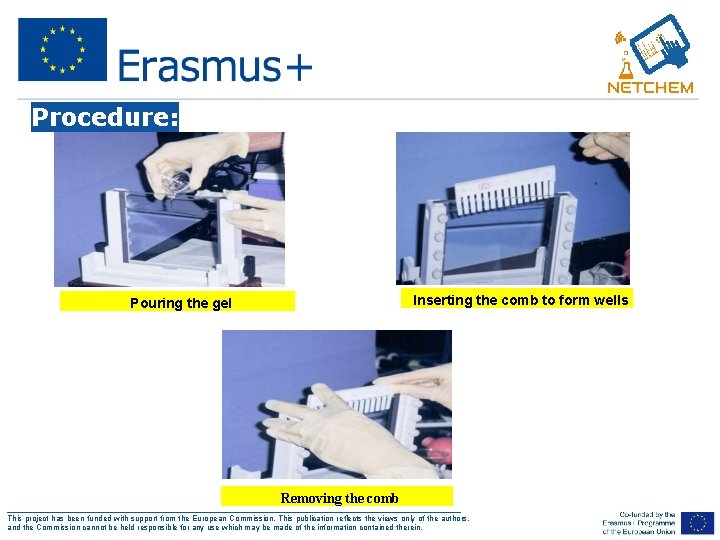
Procedure: Inserting the comb to form wells Pouring the gel Removing the comb ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Procedure: • Loading the gel q 1. Fill wells with distilled water or tank buffer as a rinse • 2. Pour off rinse and ½ fill with tank buffer • 3. Place extract into sample well using a digital pipette with gel loading tips. Sample size loaded will vary on species and gel type. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

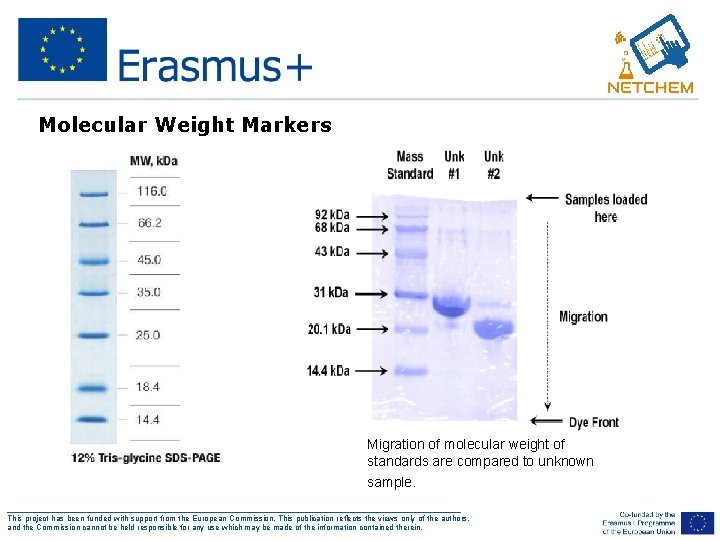
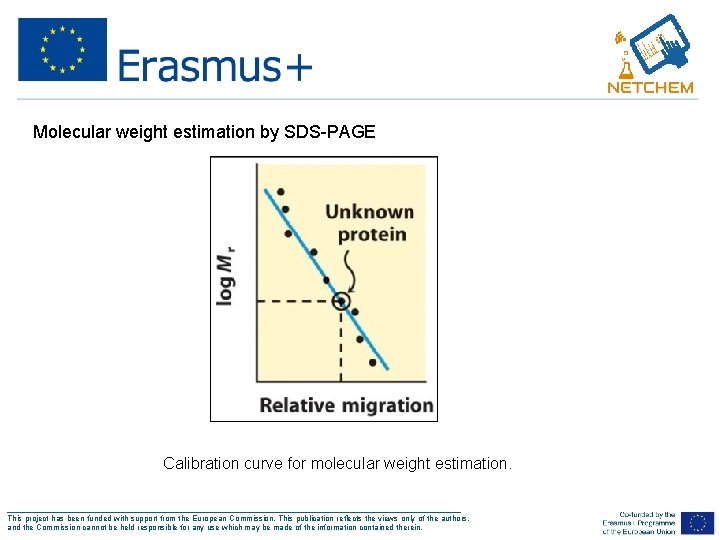
Determine Molecular Weight 1. Run standard molecular weight markers on gel 2. Run unknown protein on the same gel 3. Create a graph of the mol wt versus distance molecule has moved 4. Using the distance the unknown has moved determine the molecular weight from graph ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Molecular Weight Markers Migration of molecular weight of standards are compared to unknown sample. wt std vs unknown ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Molecular weight estimation by SDS-PAGE Calibration curve for molecular weight estimation. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

DESCRIPTION OF THE EDUCATIONAL ELEMENT Educational element title Educational field Level of study Evaluation of wheat genotypes by polyacrilamide gel electrophoresis Chemistry Master academic studies Food Chemical Analysis Title of course in which Educational element is implemented (lecture or lab exercise) Title of teaching unit Teacher Target group (study program, year of study) Polyacrylamide Gel Electrophoresis (PAGE) of Proteins Ph. D Dhurata Feta, Ph. D Artiona Laze Master Study Programme –Food Analysis, 1 st year The chemist show the principle of SDS-PAGE and how to determine protein molecular weight Educational objectives of educational element Required preliminary knowledge and skills Material available at Moodle platform for the educational element: -Type (. mp 4/. avi/. ppt/. pdf/. doc/. jpeg …): -Size (MB): -Used language in the material: Knowledge's of Instrumental Methods of Analyses Video clip (. mp 4) 16 MB english ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. No

DESCRIPTION OF REMOTE ACCESS 1. NETCHEM COMMUNICATION SIDES (NOTE: NETCHEM Communication is defined as event that involves all kinds of internet interactions (in real time and not in real time) between participants via devices (PCs, laptops, tablets andmobilephones)) host side (NOTE: Host side of NETCHEM Communication is defined as PC who invites other users to join the session) guest side (NOTE: Guest side of NETCHEM Communication is defined as PC who joins the invitation to session) 1. COMMUNICATION SOFWARE Meeting: Remote control: Team Viewer Meeting and Remote control simultaneously: Skype Call 1: 1: Conference Call: 1. COMUNICATION HARDWARE on host side on guest side 1. INFORMATION EXCHANGE TYPE Educational (one side is dominantly receptive) Place of Educator participant: Number of educator(s): Place of student participant: Number of student participant(s): Consultative Number of host side participant(s): (two sides are equal in giving-receiving Number of guest side participant(s): information) ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

Author, Editor and Referee References This remote access laboratory was created thanks to work done primarily at University of Niš. Contributors to this material were: Ph. D. Dhurata Feta, Ph. D. Artiona Laze, full professors Refereeing of this material was done by: ___________ Editing into NETCHEM Format and onto NETCHEM platform was completed by: __________________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

References and Supplemental Material The NETCHEM platform was established at the University of Nis in 2016 -2019 through the Erasmus Programme. Please contact a NETCHEM representatives at your institution or visit our website for an expanded contact list. The work included had been led by the NETCHEM staff at your institution. ___________________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
 Polyacrylamide gel electrophoresis (page)
Polyacrylamide gel electrophoresis (page) Sds-page procedure flow chart
Sds-page procedure flow chart Agarose gel electrophoresis vs sds page
Agarose gel electrophoresis vs sds page Agarose gel vs polyacrylamide gel
Agarose gel vs polyacrylamide gel Ap biology lab 6
Ap biology lab 6 Selective breeding definition biology
Selective breeding definition biology Disadvantages of agarose gel electrophoresis
Disadvantages of agarose gel electrophoresis Advantages of agarose gel electrophoresis
Advantages of agarose gel electrophoresis Zone electrophoresis definition
Zone electrophoresis definition Zone electrophoresis definition
Zone electrophoresis definition Electrolysis gel
Electrolysis gel Gel electrophoresis definition
Gel electrophoresis definition Gel electrophoresis separates dna by
Gel electrophoresis separates dna by Process of gel electrophoresis
Process of gel electrophoresis Electrophoresis definition
Electrophoresis definition Gel electrophoresis separates dna by
Gel electrophoresis separates dna by Micropipette reading quiz
Micropipette reading quiz Translate
Translate Gel electrophoresis result
Gel electrophoresis result