Physical and Chemical Changes and Exothermic and Endothermic
















- Slides: 25

Physical and Chemical Changes and Exothermic and Endothermic Reactions

Physical Change ● Changes in a substance that do not alter the chemical properties of the substance ● Things about the substance change, but the substance remains the same ● For instance, an ice cube melts into water and you can boil that into a gas, but it remains H 2 O the whole time ● One or more of its physical properties may be changed ● Ex: change in size, shape, dissolving. possibly color change (as long as a new substance is not created)

Chemical Change ● Changes that alter the chemical properties of a substance ● A change that creates a different substance ● Clues: bubbles (indicates the production of a gas), getting hot or cold, color change ● Ex: iron rusting, souring of milk, burning paper

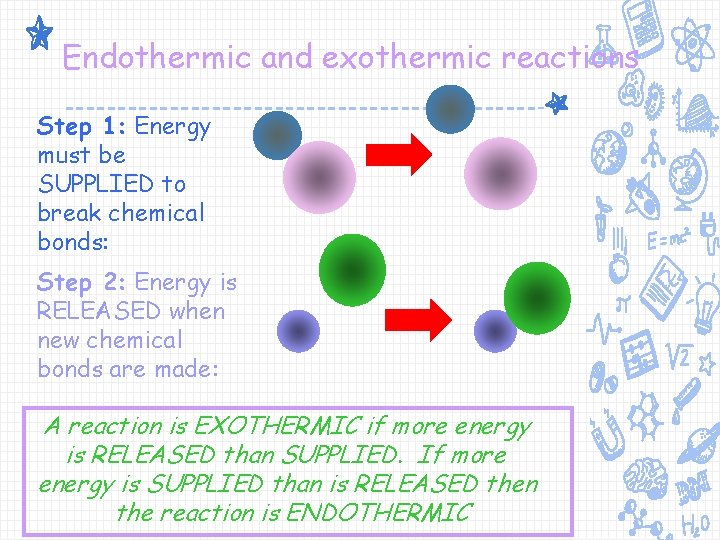
Endothermic and exothermic reactions Step 1: Energy must be SUPPLIED to break chemical bonds: Step 2: Energy is RELEASED when new chemical bonds are made: A reaction is EXOTHERMIC if more energy is RELEASED than SUPPLIED. If more energy is SUPPLIED than is RELEASED then the reaction is ENDOTHERMIC


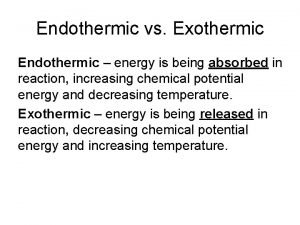
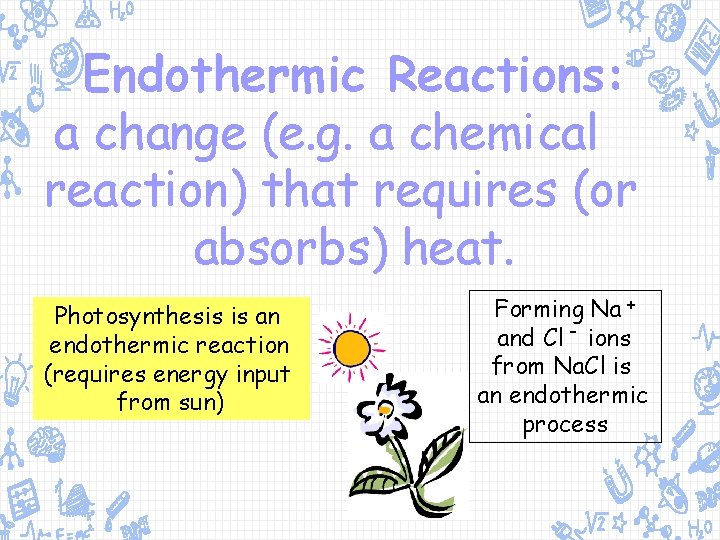
Endothermic Reactions: a change (e. g. a chemical reaction) that requires (or absorbs) heat. Photosynthesis is an endothermic reaction (requires energy input from sun) Forming Na + and Cl - ions from Na. Cl is an endothermic process

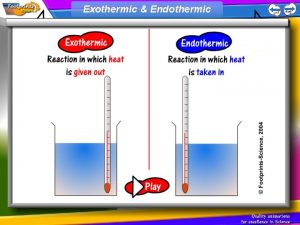
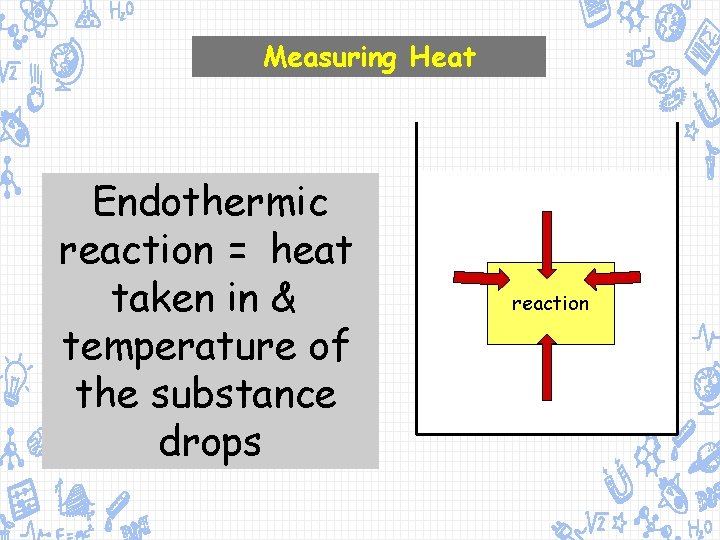
Measuring Heat Endothermic reaction = heat taken in & temperature of the substance drops reaction

Ammonium nitrate + water

Starting temperature 20⁰ C

Add ammonium nitrate to water

ending temperature of 12⁰ C a temperature decrease of 8⁰ C


Activation energy Energy given out by reaction

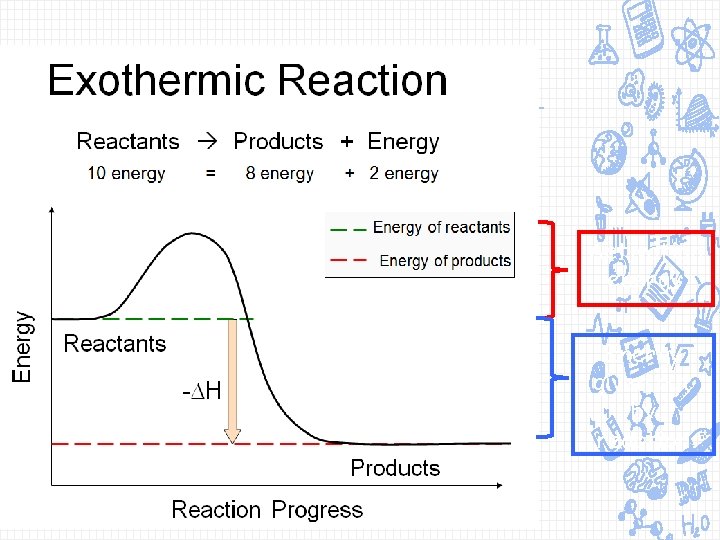
Exothermic Reaction: a change (e. g. a chemical reaction) that releases heat. Burning fossil fuels is an exothermic reaction

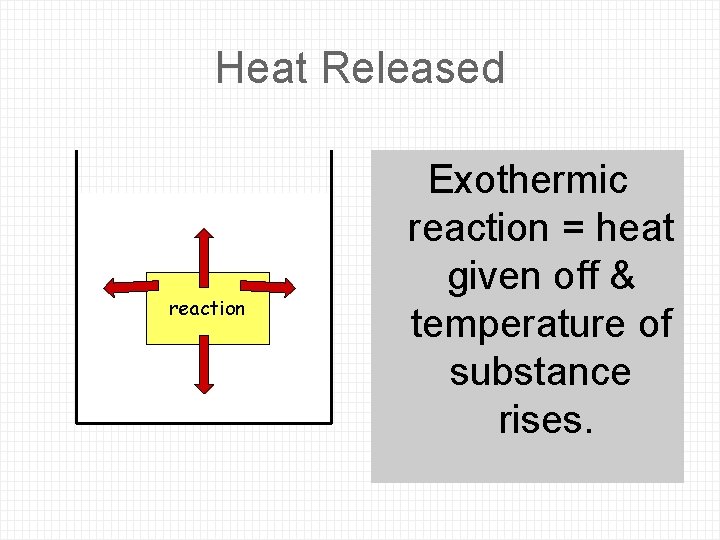
Heat Released reaction Exothermic reaction = heat given off & temperature of substance rises.

Exothermic vs endothermic: EXOTHERMIC – more energy is given out than is taken in (e. g. burning, respiration) © Teachable. Some rights reserved. http: //teachable. net/res. asp? r=1910 ENDOTHERMIC – energy is taken in but not necessarily given out (e. g. photosynthesis)


Examples Exothermic • Combustion of fuels • Evaporation of Water • Yeast & Hydrogen Peroxide • Epsom salts & water © Teachable. Some rights reserved. http: //teachable. net/res. asp? r=1910 Endothermic • • Photosynthesis Acetic Acid & Sodium Bicarbonate Cold Packs Melting Ice

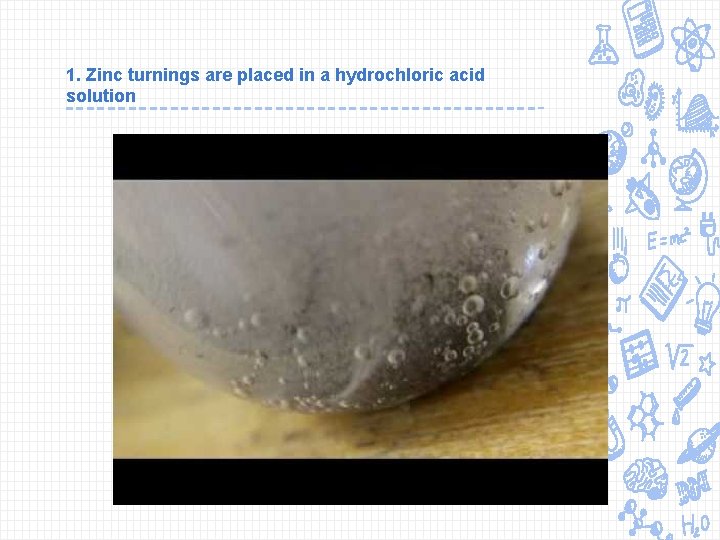
1. Zinc turnings are placed in a hydrochloric acid solution

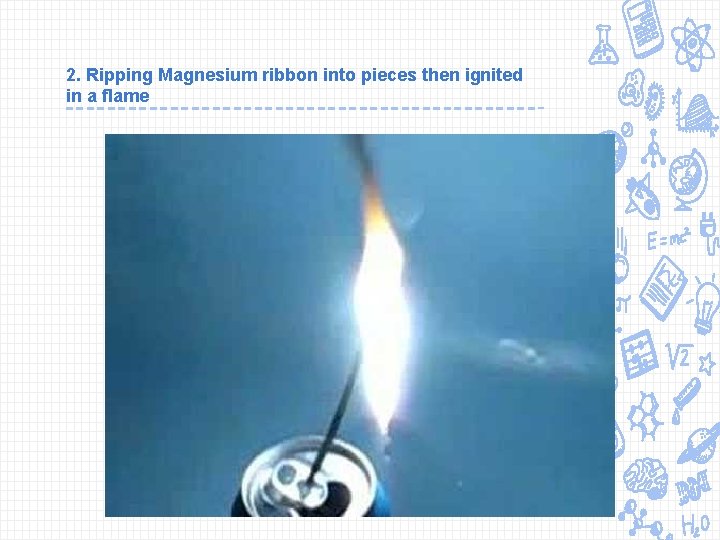
2. Ripping Magnesium ribbon into pieces then ignited in a flame

3. A penny is coated with zinc then heated Normal Penny Coated with Zinc After being heated

4. Polymers A and B are mixed

5. Hexane is poured down ramp and ignited

6. A styrofoam cup in acetone

7. Gas bubbles are made, then ignited
 Sample of chemical changes
Sample of chemical changes Endothermic and exothermic reaction experiment grade 11
Endothermic and exothermic reaction experiment grade 11 Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Define exothermic reaction
Define exothermic reaction Vaporization endothermic or exothermic
Vaporization endothermic or exothermic Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic 6 phase changes
6 phase changes How to determine exothermic or endothermic
How to determine exothermic or endothermic Ectotherms
Ectotherms Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Ins
Ins Exothermic or endothermic
Exothermic or endothermic Endothermic vs exothermix
Endothermic vs exothermix Particles of condensation
Particles of condensation Endothermic energy
Endothermic energy Is propane burning in a grill endothermic or exothermic
Is propane burning in a grill endothermic or exothermic Endo vs exo graphs
Endo vs exo graphs Is a firecracker exploding endothermic or exothermic
Is a firecracker exploding endothermic or exothermic Is photosynthesis endothermic
Is photosynthesis endothermic System vs surroundings
System vs surroundings Phase change diagram endothermic exothermic
Phase change diagram endothermic exothermic Compare and contrast chemical and physical changes
Compare and contrast chemical and physical changes Section 1 chemical changes
Section 1 chemical changes