PHY 102 Quantum Physics Topic 1 The nature




- Slides: 42

PHY 102: Quantum Physics Topic 1 The nature of light

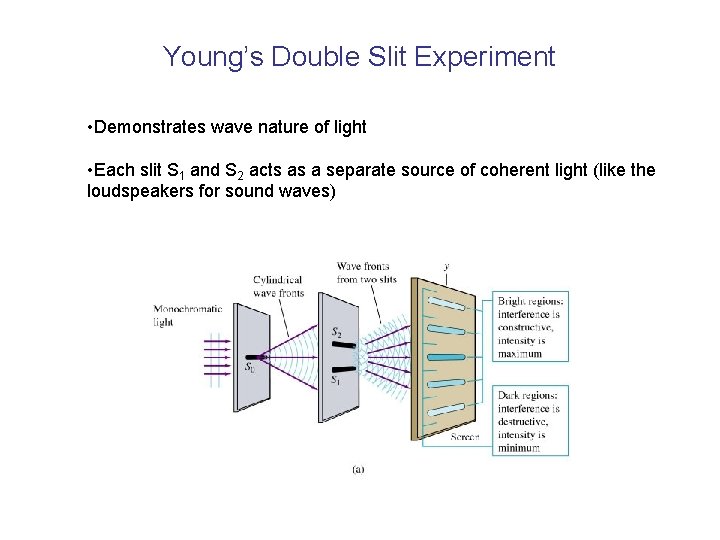
Young’s Double Slit Experiment • Demonstrates wave nature of light • Each slit S 1 and S 2 acts as a separate source of coherent light (like the loudspeakers for sound waves)

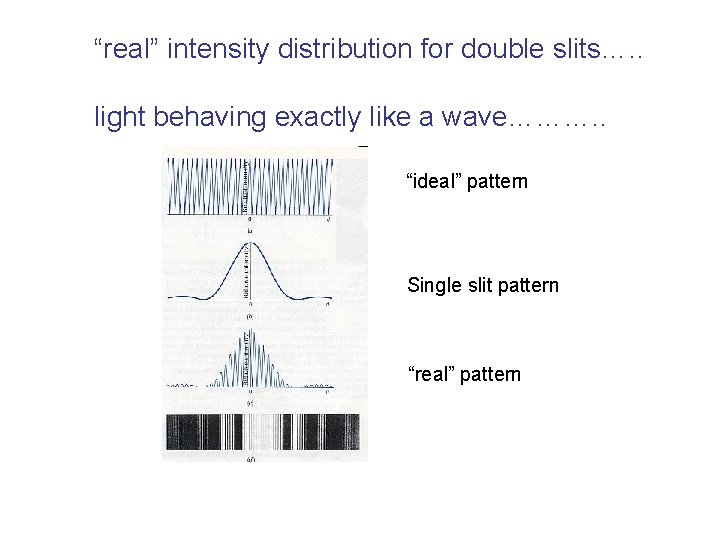
“real” intensity distribution for double slits…. . light behaving exactly like a wave………. . “ideal” pattern Single slit pattern “real” pattern

What kind of wave (what’s waving? ) • Key point: propagation of a light wave does NOT require a medium • Light can propagate through empty space (vacuum) Consider two of MAXWELL’S EQUATIONS OF ELECTROMAGNETISM: (essentially Faraday’s law and Ampere’s law, respectively)

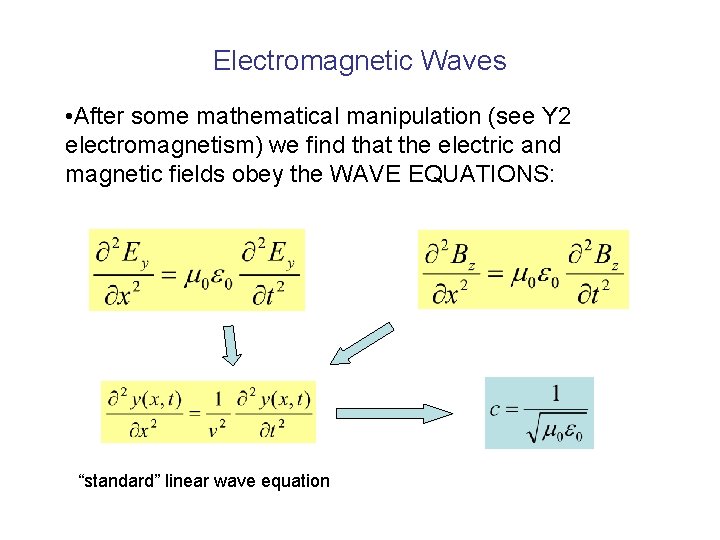
Electromagnetic Waves • After some mathematical manipulation (see Y 2 electromagnetism) we find that the electric and magnetic fields obey the WAVE EQUATIONS: “standard” linear wave equation

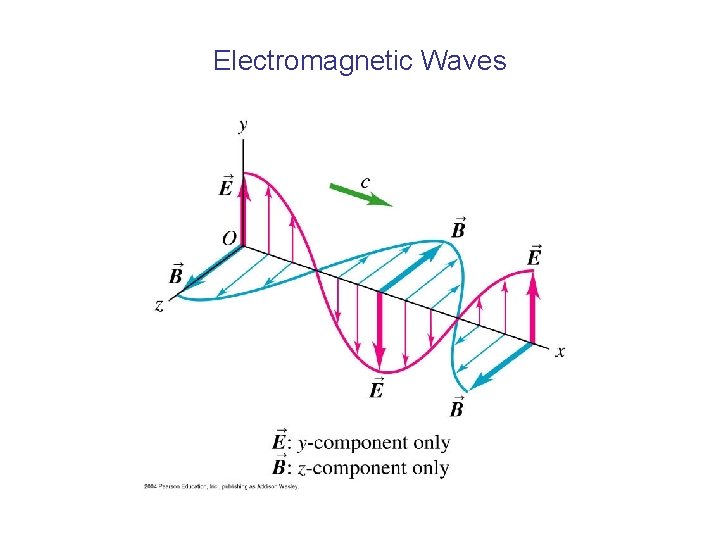
Electromagnetic Waves

Energy in Electromagnetic Waves • For a wave, intensity = energy flow through unit area per unit time = P/A • In the case of an EM wave, intensity corresponds to the “brightness” of the radiation • Studies of the PHOTOELECTRIC EFFECT by Lennard (~1900) gave results that could not be explained by the classical wave picture of light………

The photoelectric effect • In a metal, the flow of electric current is due to the flow of charged particles called electrons • The electrons are relatively free to move throughout the metal (electron “gas” or “sea”), so most metals are good conductors of electricity. • By shining light on a metal, some of these electrons can be “knocked out” of the metal to generate an electric current outside of the metal • Although basic photoelectric effect can be explained with classical wave model of light, the detailed results of such an experiment CAN NOT!. . .

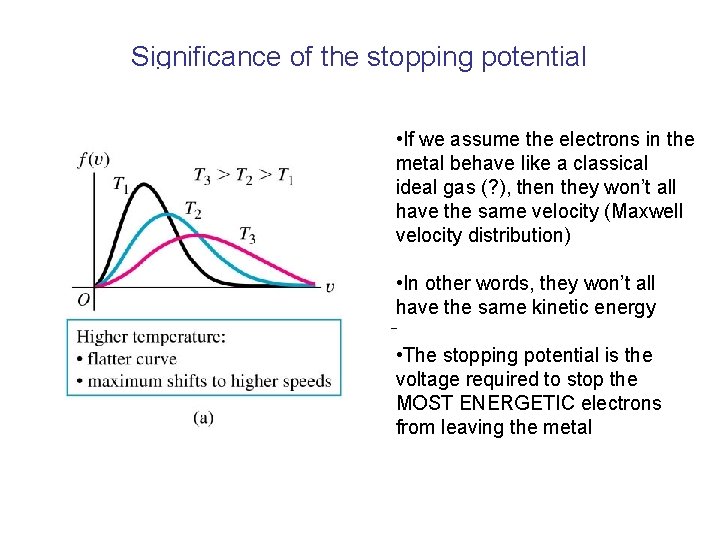
Electrons in metals • Electrical current arises from the flow of charged particles, usually electrons. • In the “Drude model” of a metal, there are many free electrons, which behave like an ideal gas of charged particles. • Because of this large number of free, charged particles, metals are usually very good electrical conductors. • If electrons in a metal behave as an ideal gas, we expect them to have a spread of kinetic energies………………….

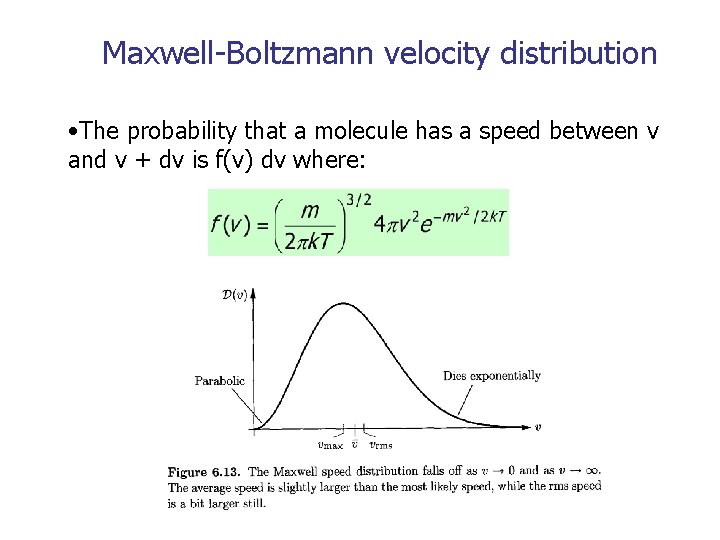
Maxwell-Boltzmann velocity distribution • The probability that a molecule has a speed between v and v + dv is f(v) dv where:

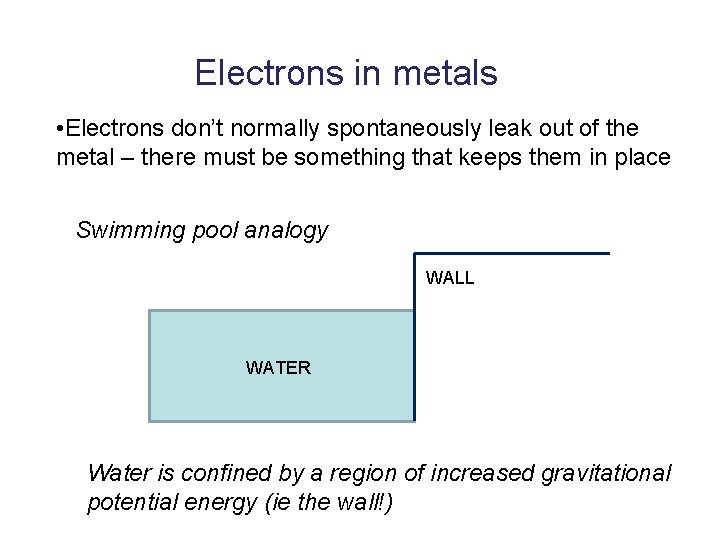
Electrons in metals • Electrons don’t normally spontaneously leak out of the metal – there must be something that keeps them in place Swimming pool analogy WALL WATER Water is confined by a region of increased gravitational potential energy (ie the wall!)

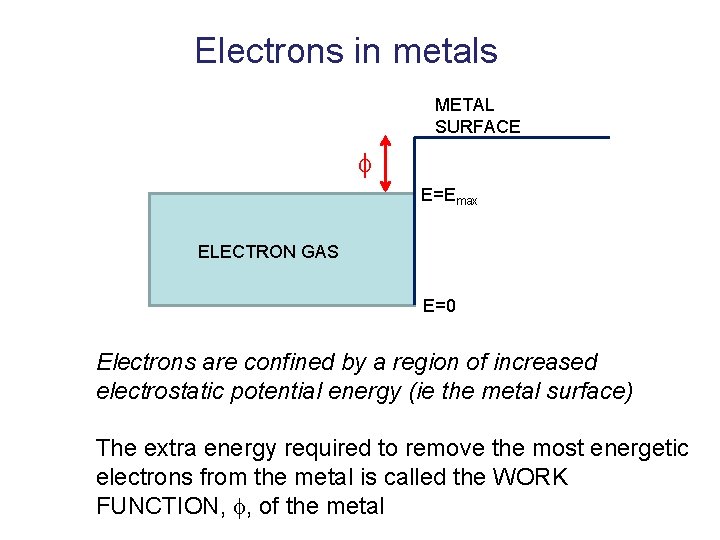
Electrons in metals METAL SURFACE E=Emax ELECTRON GAS E=0 Electrons are confined by a region of increased electrostatic potential energy (ie the metal surface) The extra energy required to remove the most energetic electrons from the metal is called the WORK FUNCTION, , of the metal

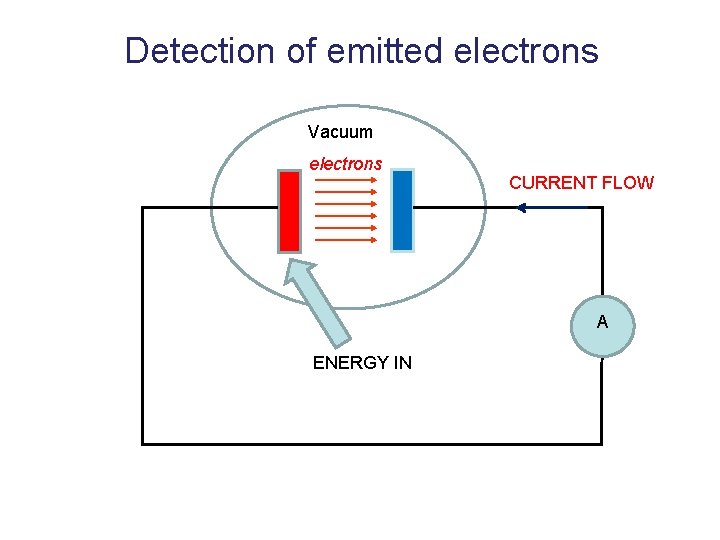
Detection of emitted electrons Vacuum A

Detection of emitted electrons Vacuum electrons CURRENT FLOW A ENERGY IN

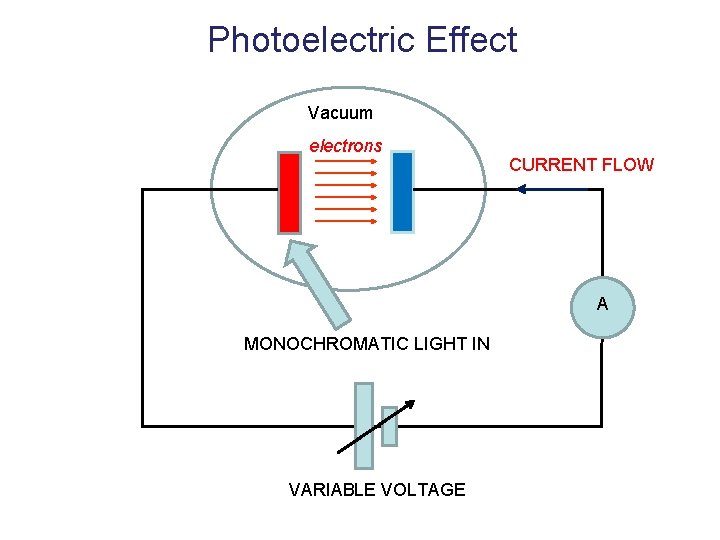
Photoelectric Effect Vacuum electrons CURRENT FLOW A MONOCHROMATIC LIGHT IN VARIABLE VOLTAGE

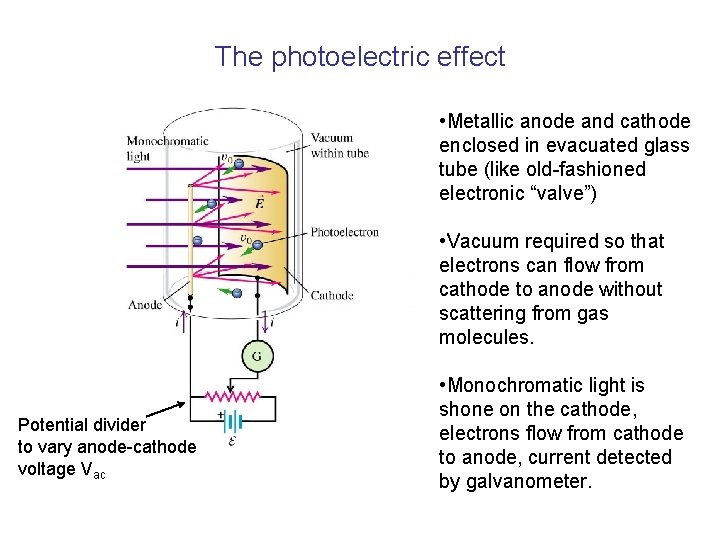
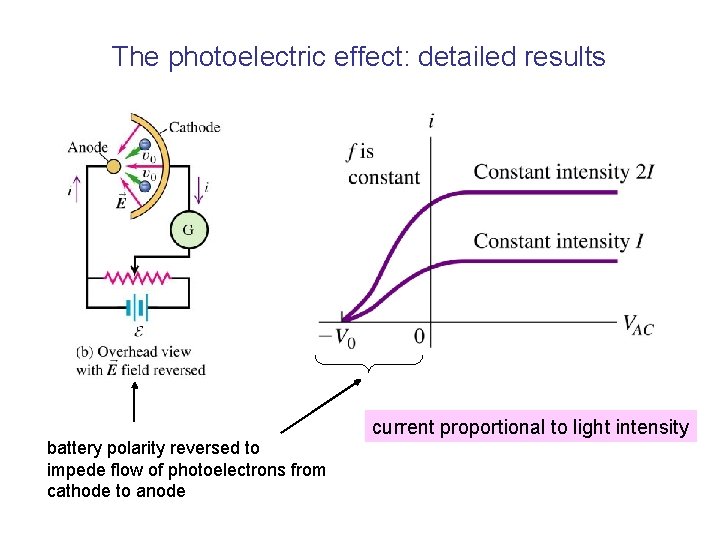
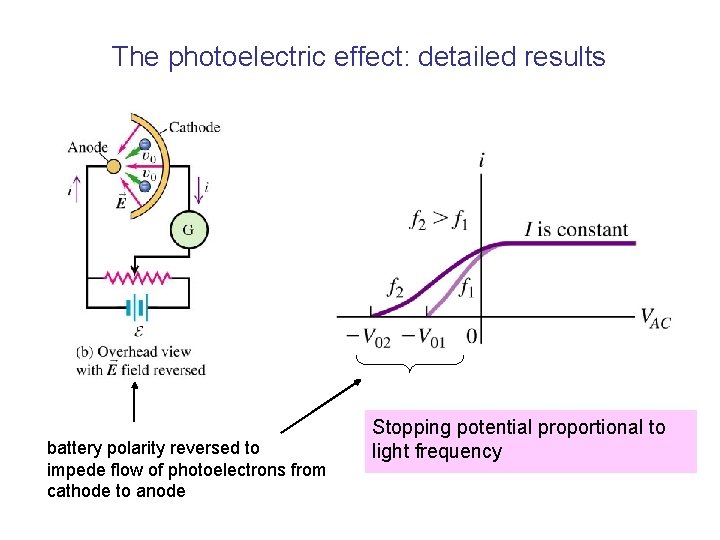
The photoelectric effect • Metallic anode and cathode enclosed in evacuated glass tube (like old-fashioned electronic “valve”) • Vacuum required so that electrons can flow from cathode to anode without scattering from gas molecules. Potential divider to vary anode-cathode voltage Vac • Monochromatic light is shone on the cathode, electrons flow from cathode to anode, current detected by galvanometer.

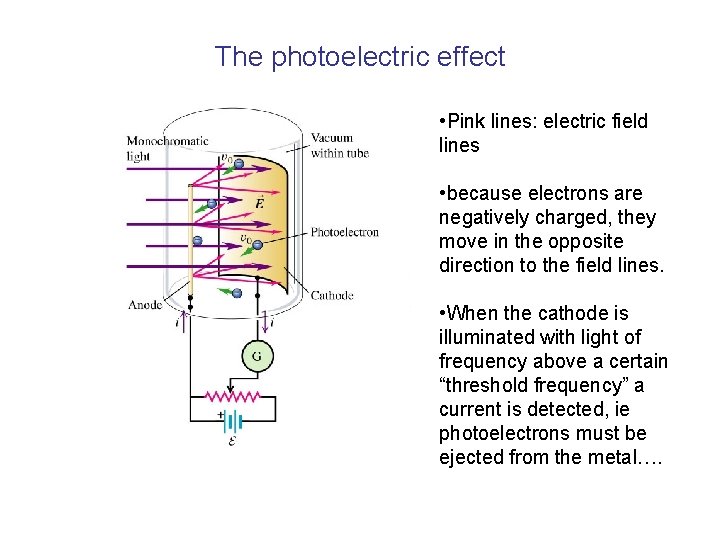
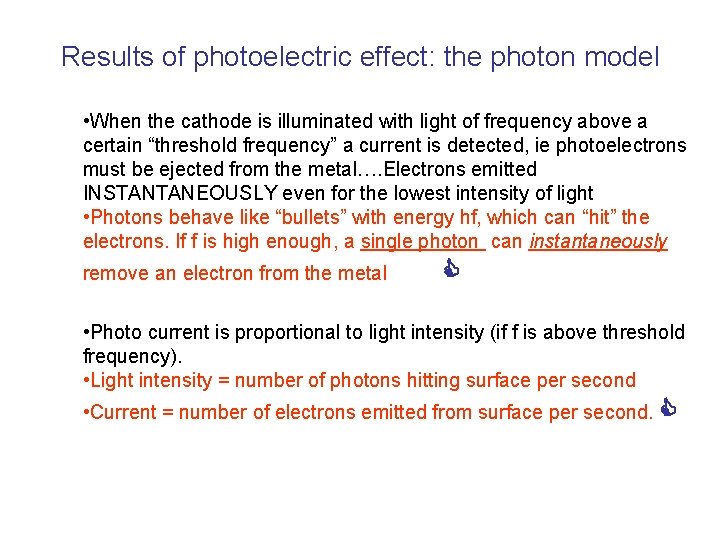
The photoelectric effect • Pink lines: electric field lines • because electrons are negatively charged, they move in the opposite direction to the field lines. • When the cathode is illuminated with light of frequency above a certain “threshold frequency” a current is detected, ie photoelectrons must be ejected from the metal….

The photoelectric effect • When the cathode is illuminated with light of frequency above a certain “threshold frequency” a current is detected, ie photoelectrons must be ejected from the metal…. Electrons emitted INSTANTANEOUSLY even for the lowest intensity of light • If the frequency of the incident light is below the threshold frequency, no electrons emitted, no matter how intense the light is. • If light were behaving entirely like a classical EM wave, its power would be proportional to intensity, but independent of frequency (mechanical wave energy proportional to amplitude and frequency, EM wave energy proportional to amplitude (intensity) only) • Would expect intense light of any frequency to generate photoelectrons, and also “time delay” for photoemission due to light of low intensity…………. .

The photoelectric effect: detailed results battery polarity reversed to impede flow of photoelectrons from cathode to anode current proportional to light intensity

The photoelectric effect: detailed results battery polarity reversed to impede flow of photoelectrons from cathode to anode Stopping potential proportional to light frequency

Significance of the stopping potential • If we assume the electrons in the metal behave like a classical ideal gas (? ), then they won’t all have the same velocity (Maxwell velocity distribution) • In other words, they won’t all have the same kinetic energy • The stopping potential is the voltage required to stop the MOST ENERGETIC electrons from leaving the metal

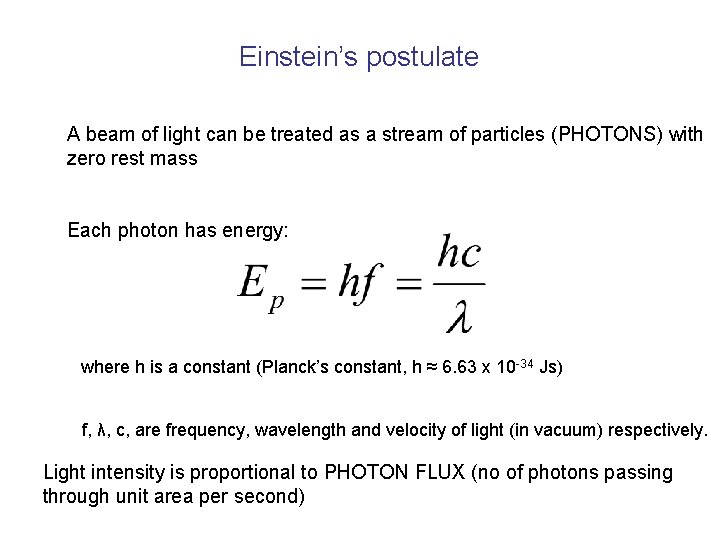
Einstein’s postulate A beam of light can be treated as a stream of particles (PHOTONS) with zero rest mass Each photon has energy: where h is a constant (Planck’s constant, h ≈ 6. 63 x 10 -34 Js) f, λ, c, are frequency, wavelength and velocity of light (in vacuum) respectively. Light intensity is proportional to PHOTON FLUX (no of photons passing through unit area per second)

Results of photoelectric effect: the photon model • When the cathode is illuminated with light of frequency above a certain “threshold frequency” a current is detected, ie photoelectrons must be ejected from the metal…. Electrons emitted INSTANTANEOUSLY even for the lowest intensity of light • Photons behave like “bullets” with energy hf, which can “hit” the electrons. If f is high enough, a single photon can instantaneously remove an electron from the metal • Photo current is proportional to light intensity (if f is above threshold frequency). • Light intensity = number of photons hitting surface per second • Current = number of electrons emitted from surface per second.

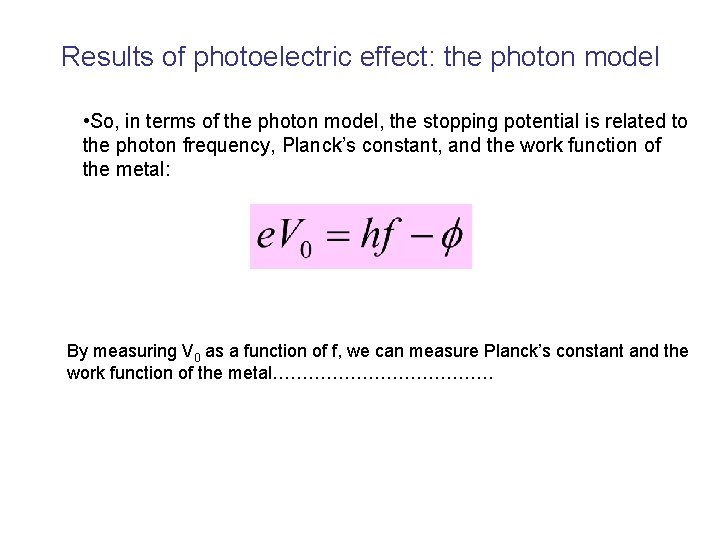
Results of photoelectric effect: the photon model • So, in terms of the photon model, the stopping potential is related to the photon frequency, Planck’s constant, and the work function of the metal: By measuring V 0 as a function of f, we can measure Planck’s constant and the work function of the metal……………….

Example Calculation 1 • In a photoelectric experiment a reverse potential of 2 V is required to stop the flow of current for light of a certain frequency. Calculate a) the maximum kinetic energy and b) the maximum velocity of the emitted photoelectrons……. .

The Compton Effect

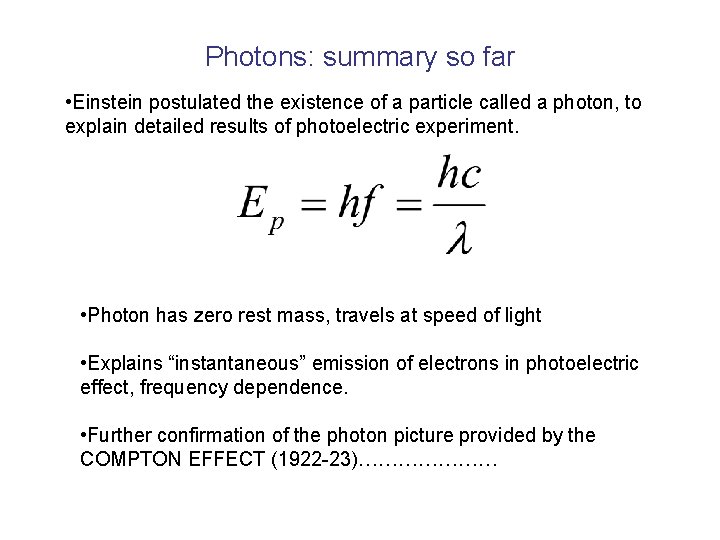
Photons: summary so far • Einstein postulated the existence of a particle called a photon, to explain detailed results of photoelectric experiment. • Photon has zero rest mass, travels at speed of light • Explains “instantaneous” emission of electrons in photoelectric effect, frequency dependence. • Further confirmation of the photon picture provided by the COMPTON EFFECT (1922 -23)…………………

A reminder about relativity…… • Einstein’s Special Theory of Relativity, 1905: • The laws of physics are the same in every inertial frame of reference (in which Newton’s first law is valid) • The speed of light in a vacuum is the same in all inertial frames of reference, and is independent of the motion of the source. (corollary: the velocity of light can’t be exceeded) Many important consequences: length contraction, time dilation effects at high speeds, mass/energy equivalence……

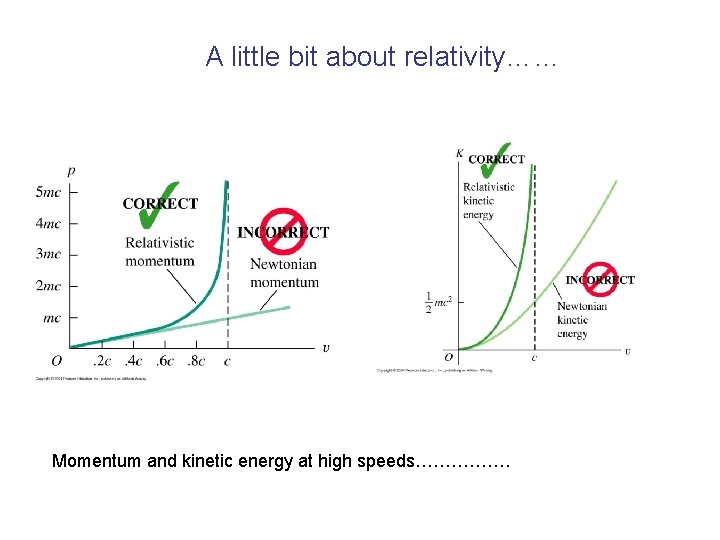
A little bit about relativity…… Momentum and kinetic energy at high speeds…………….

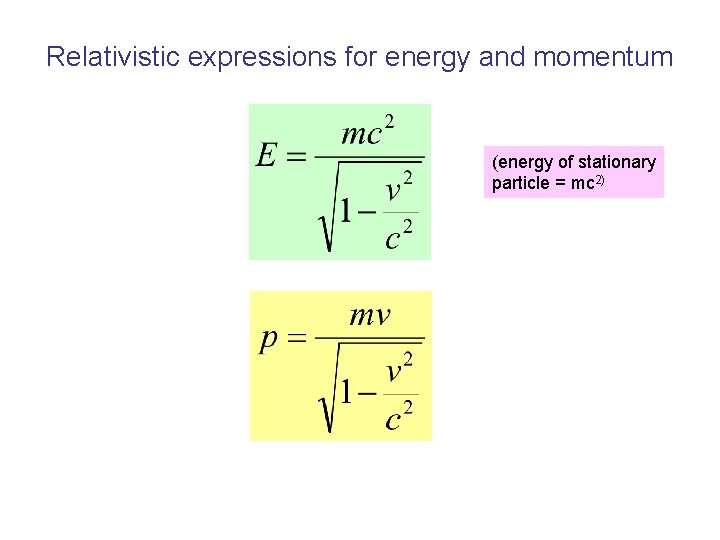
Relativistic expressions for energy and momentum (energy of stationary particle = mc 2)

Relativistic expressions for energy and momentum

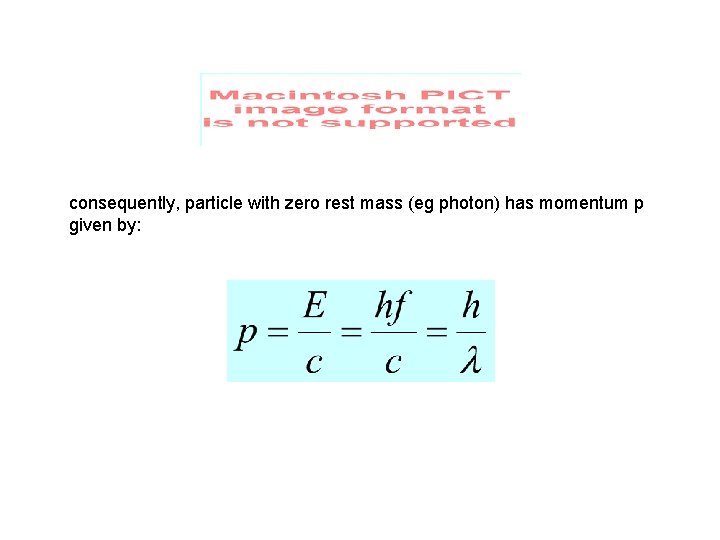
consequently, particle with zero rest mass (eg photon) has momentum p given by:

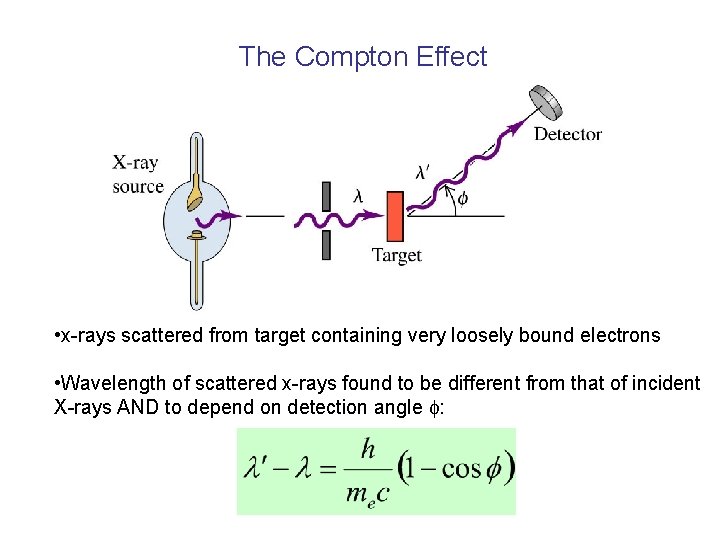
The Compton Effect • x-rays scattered from target containing very loosely bound electrons • Wavelength of scattered x-rays found to be different from that of incident X-rays AND to depend on detection angle :

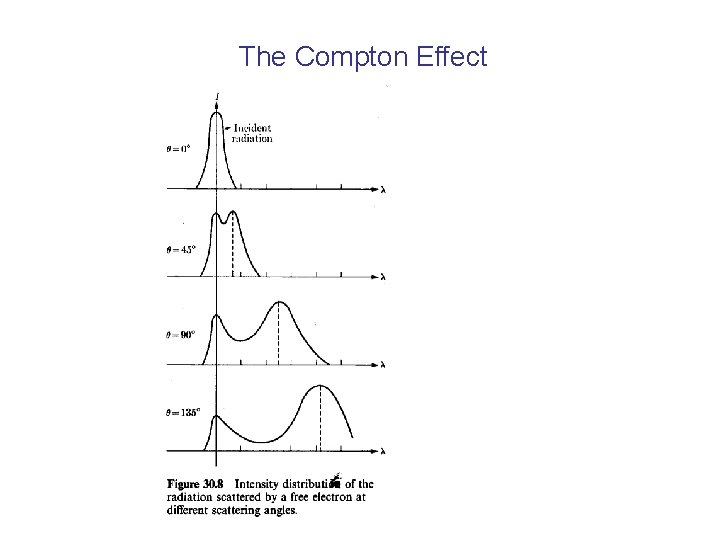
The Compton Effect

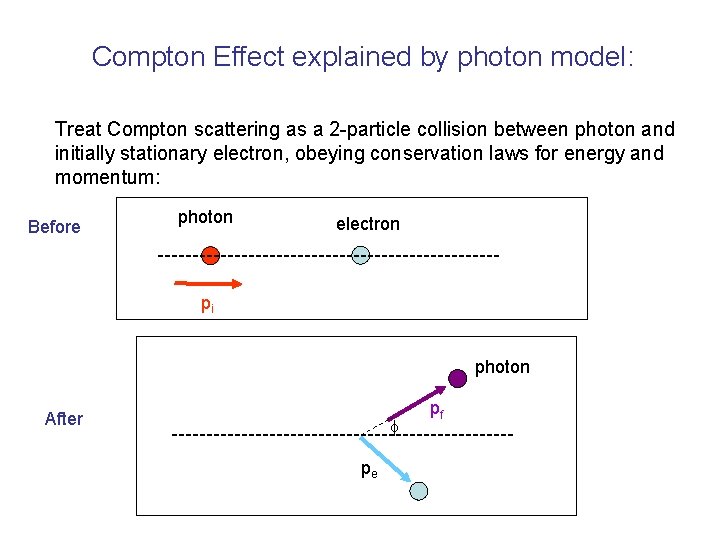
Compton Effect explained by photon model: Treat Compton scattering as a 2 -particle collision between photon and initially stationary electron, obeying conservation laws for energy and momentum: Before photon electron pi photon After pe pf

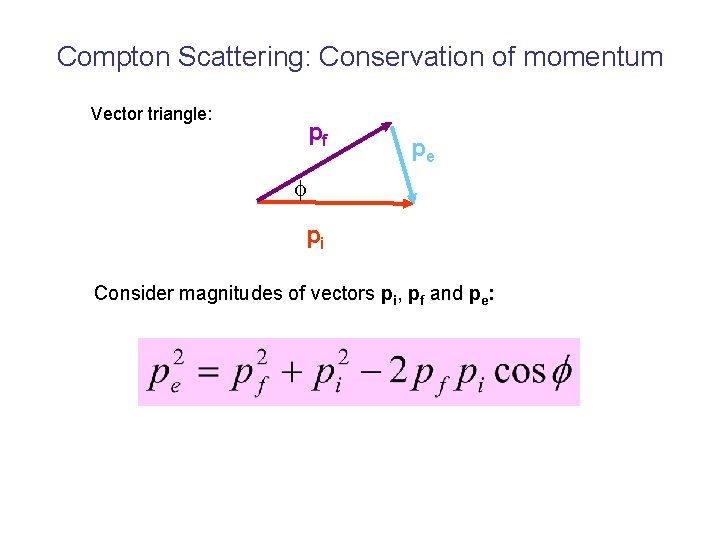
Compton Scattering: Conservation of momentum Vector triangle: pf pe pi Consider magnitudes of vectors pi, pf and pe:

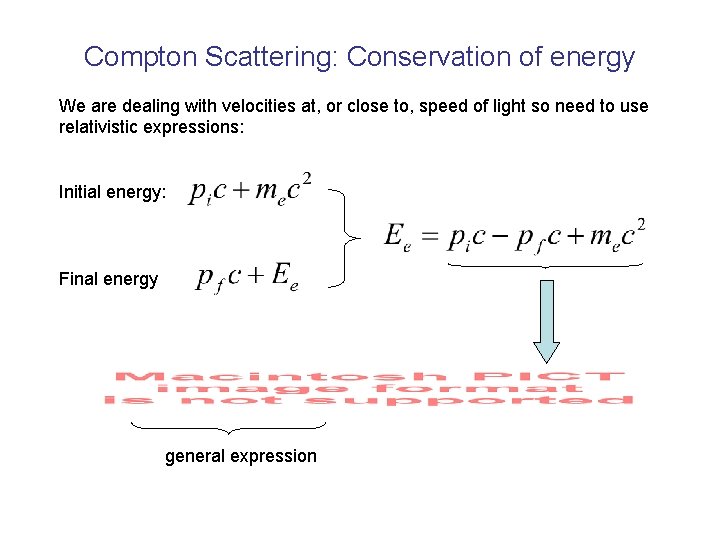
Compton Scattering: Conservation of energy We are dealing with velocities at, or close to, speed of light so need to use relativistic expressions: Initial energy: Final energy general expression

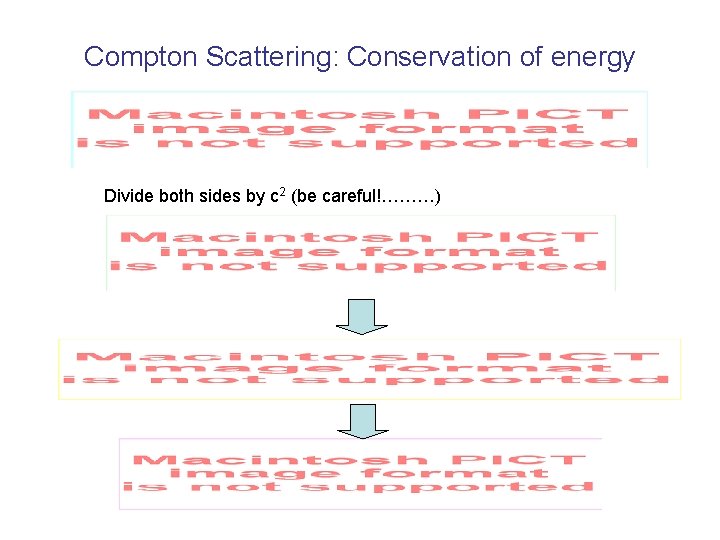
Compton Scattering: Conservation of energy Divide both sides by c 2 (be careful!………)

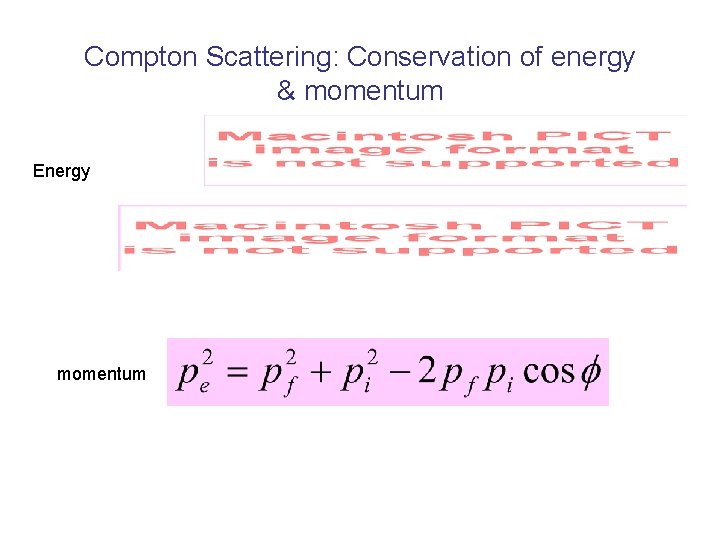
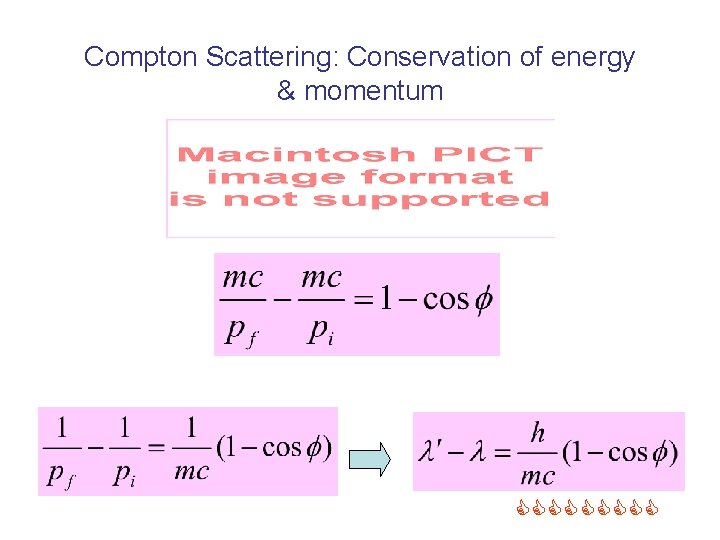
Compton Scattering: Conservation of energy & momentum Energy momentum

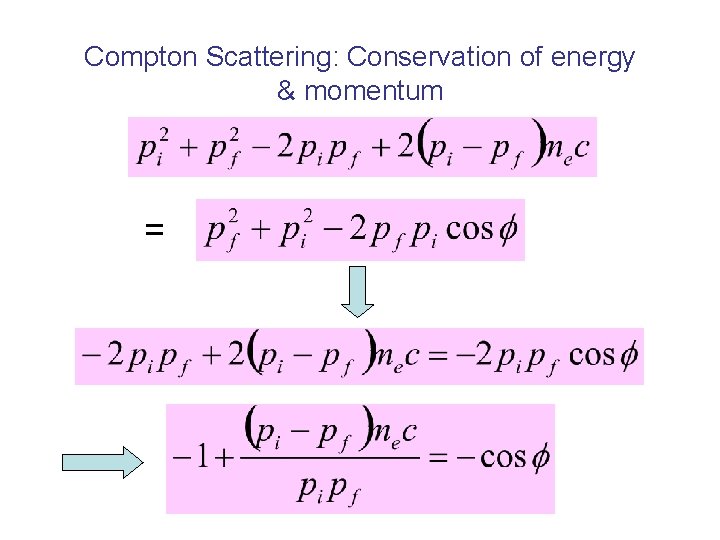
Compton Scattering: Conservation of energy & momentum =

Compton Scattering: Conservation of energy & momentum

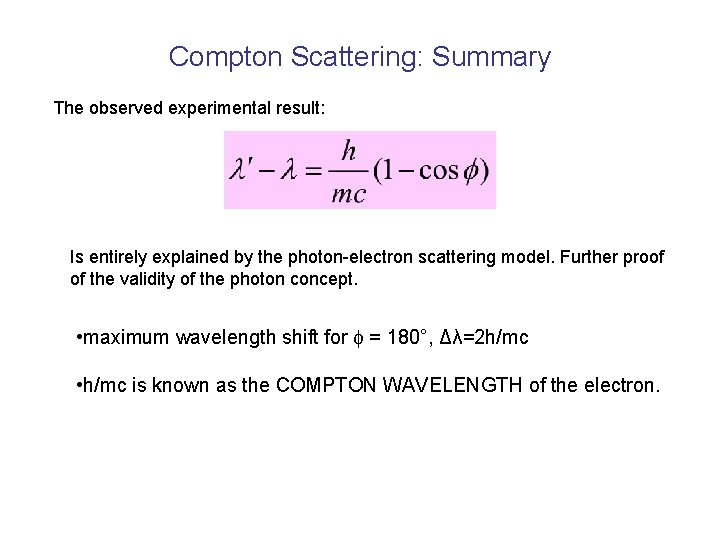
Compton Scattering: Summary The observed experimental result: Is entirely explained by the photon-electron scattering model. Further proof of the validity of the photon concept. • maximum wavelength shift for = 180°, Δλ=2 h/mc • h/mc is known as the COMPTON WAVELENGTH of the electron.