Percent Evaluation Attendance and Class Participation 5 Assignments


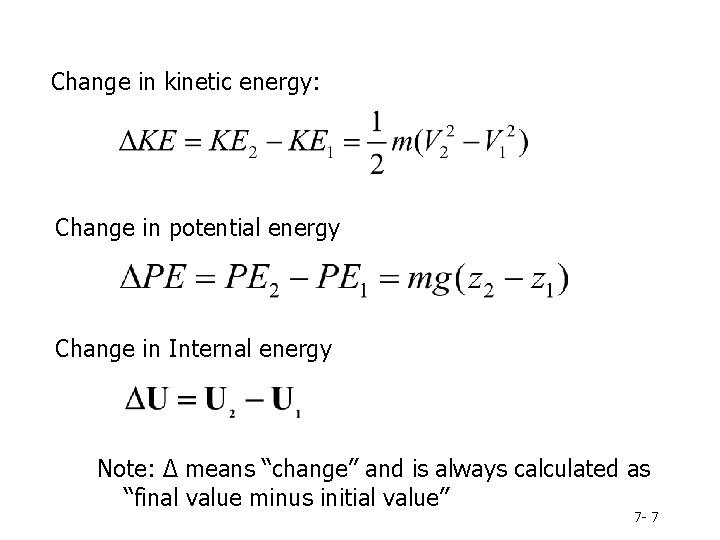








- Slides: 94

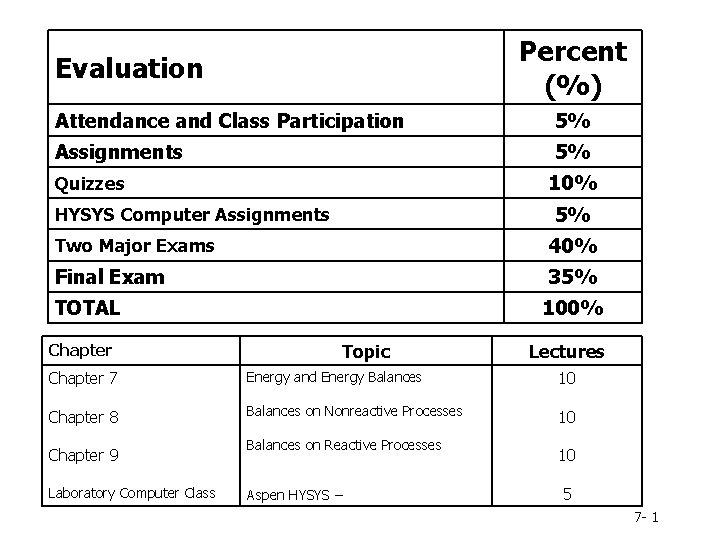
Percent (%) Evaluation Attendance and Class Participation 5% Assignments 5% 10% Quizzes 5% HYSYS Computer Assignments Two Major Exams 40% Final Exam 35% TOTAL Chapter 100% Topic Lectures Chapter 7 Energy and Energy Balances 10 Chapter 8 Balances on Nonreactive Processes 10 Chapter 9 Laboratory Computer Class Balances on Reactive Processes Aspen HYSYS – 10 5 7 - 1

Major Exam I: 28 Feb 2013, 10: 00 AM Major Exam II: 4 April 2013, 10: 00 AM Final Exam: homepage 7 - 2

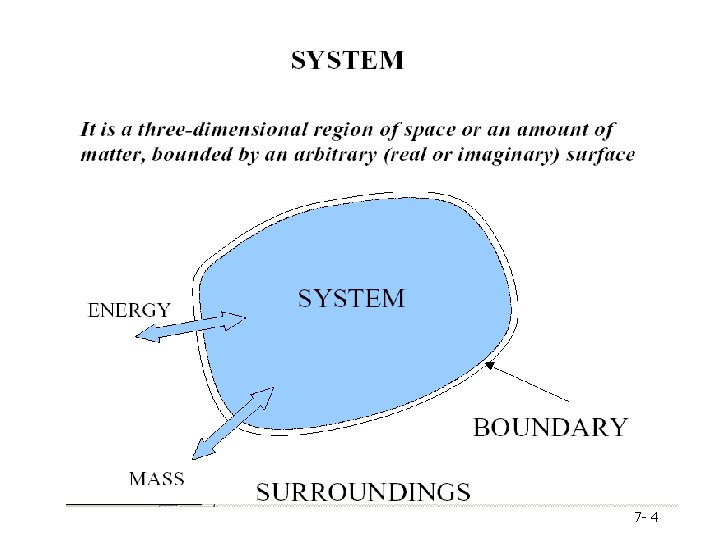
ENERGY BALANCE (Ch. E 202) Chapter 7 Energy & Energy Balances Dr. Shamsuzzoha, Ph. D 7 - 3

7 - 4

7 - 5

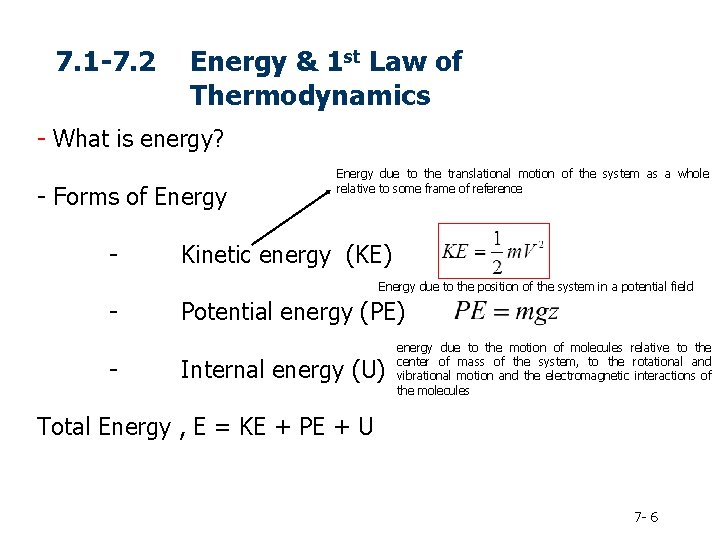
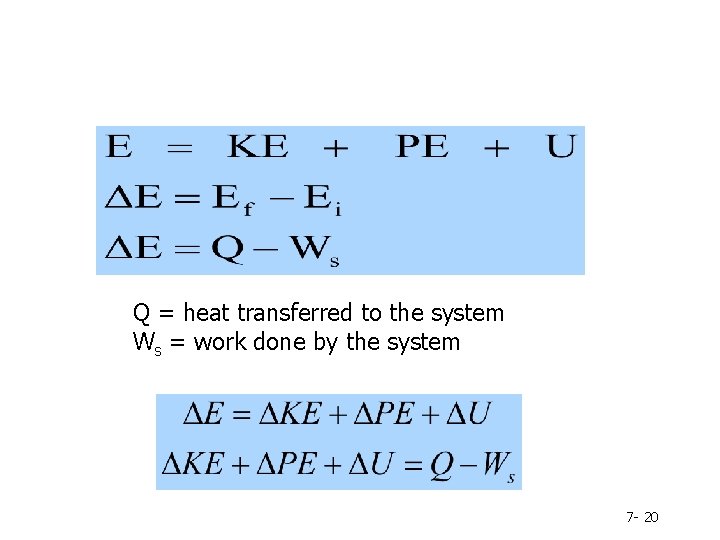
7. 1 -7. 2 Energy & 1 st Law of Thermodynamics - What is energy? - Forms of Energy - Energy due to the translational motion of the system as a whole relative to some frame of reference Kinetic energy (KE) Energy due to the position of the system in a potential field - Potential energy (PE) Internal energy (U) energy due to the motion of molecules relative to the center of mass of the system, to the rotational and vibrational motion and the electromagnetic interactions of the molecules Total Energy , E = KE + PE + U 7 - 6
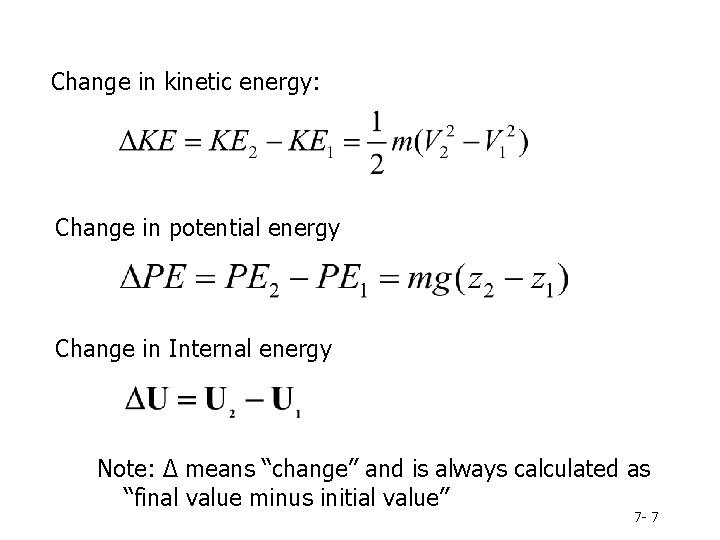
Change in kinetic energy: Change in potential energy Change in Internal energy Note: Δ means “change” and is always calculated as “final value minus initial value” 7 - 7

TEST YOURSELF 1. What forms of energy may a system possess? In what forms may energy be transferred to and from a closed system? 2. Kinetic, potential, internal; heat, work 2. Why is it meaningless to speak of the heat possessed by a system? Heat is only defined in terms of energy being transferred. 3. Suppose the initial energy of a system (internal + kinetic + potential) is Ej, the final energy is Ef , an amount of energy Q is transferred from the environment to the system as heat, and an amount W is transferred from the system to the environment as work. According to the first law of thermodynamics, how must Ei, Ef , Q, and W be related? E; + Q - W = Ef 7 - 8

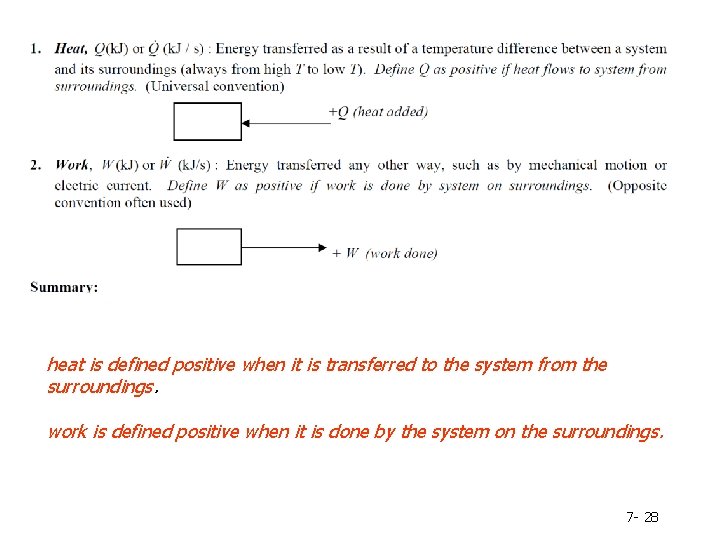
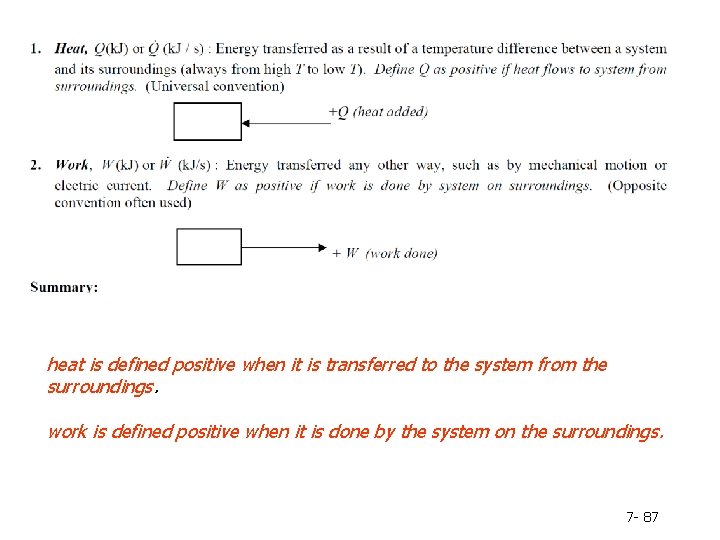
How energy can be transferred between a system and its surroundings? Heat – energy that flows as a result of temperature difference between a system and its surrounding ; heat is defined positive when it is transferred to the system from the surroundings. Work – energy that flows in response to any driving force other than a temperature difference. ; work is defined positive when it is done by the system on the surroundings. 7 - 9

Types of Work • Flow work (Wfl) - energy carried across the boundaries of a system with the mass flowing across the boundaries (i. e. internal, kinetic & potential energy) • Shaft work (Ws) - energy in transition across the boundaries of a system due to a driving force other than temperature, and not associated with mass flow (an example would be mechanical work due to a piston, pump or compressor) 7 - 10

ENERGY – CONVERSION UNITS 1 newton (N) = 1 kg. m/s 2 1 dyne = 1 g. cm/s 2 1 lbf = 32. 174 lbm. ft/s 2 7 - 11

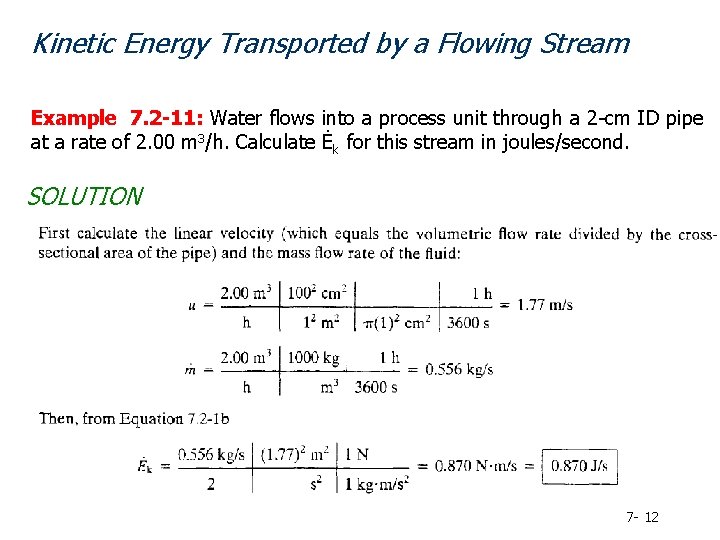
Kinetic Energy Transported by a Flowing Stream Example 7. 2 -11: Water flows into a process unit through a 2 -cm ID pipe at a rate of 2. 00 m 3/h. Calculate Ėk for this stream in joules/second. SOLUTION 7 - 12

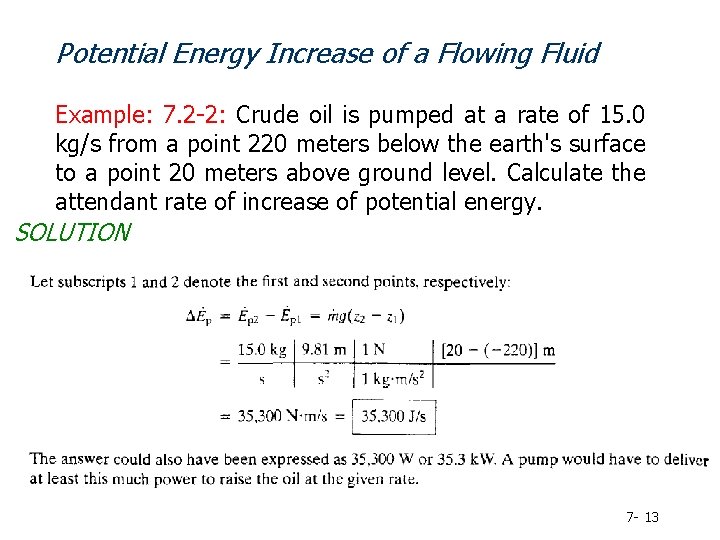
Potential Energy Increase of a Flowing Fluid Example: 7. 2 -2: Crude oil is pumped at a rate of 15. 0 kg/s from a point 220 meters below the earth's surface to a point 20 meters above ground level. Calculate the attendant rate of increase of potential energy. SOLUTION 7 - 13

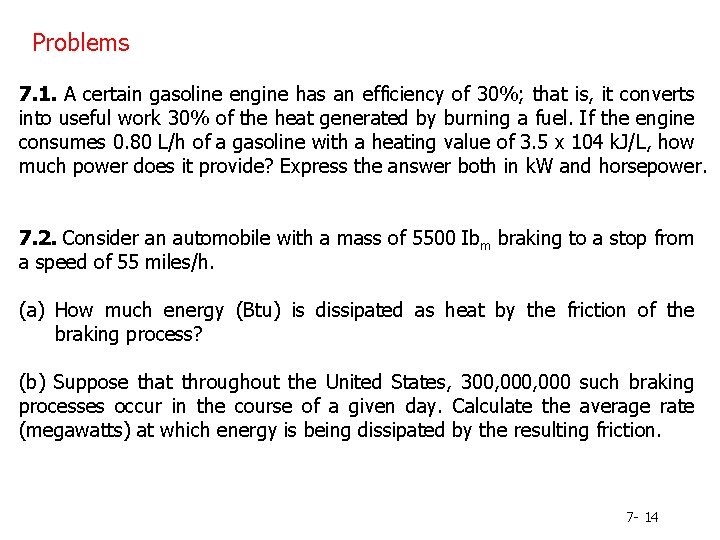
Problems 7. 1. A certain gasoline engine has an efficiency of 30%; that is, it converts into useful work 30% of the heat generated by burning a fuel. If the engine consumes 0. 80 L/h of a gasoline with a heating value of 3. 5 x 104 k. J/L, how much power does it provide? Express the answer both in k. W and horsepower. 7. 2. Consider an automobile with a mass of 5500 Ibm braking to a stop from a speed of 55 miles/h. (a) How much energy (Btu) is dissipated as heat by the friction of the braking process? (b) Suppose that throughout the United States, 300, 000 such braking processes occur in the course of a given day. Calculate the average rate (megawatts) at which energy is being dissipated by the resulting friction. 7 - 14

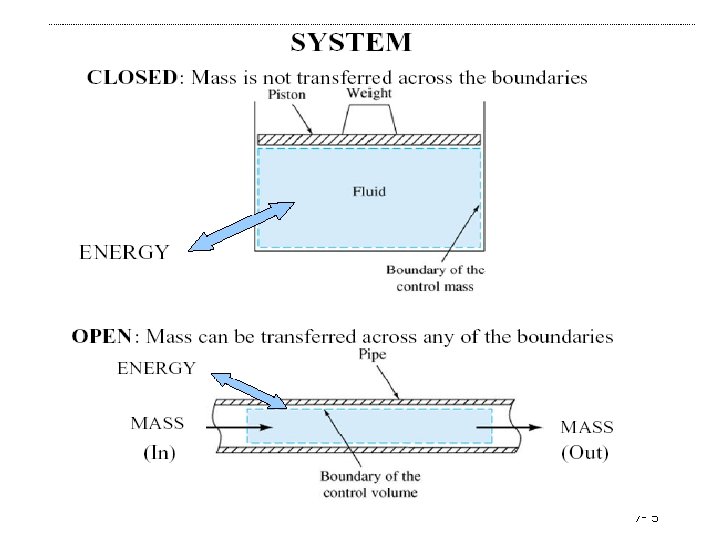
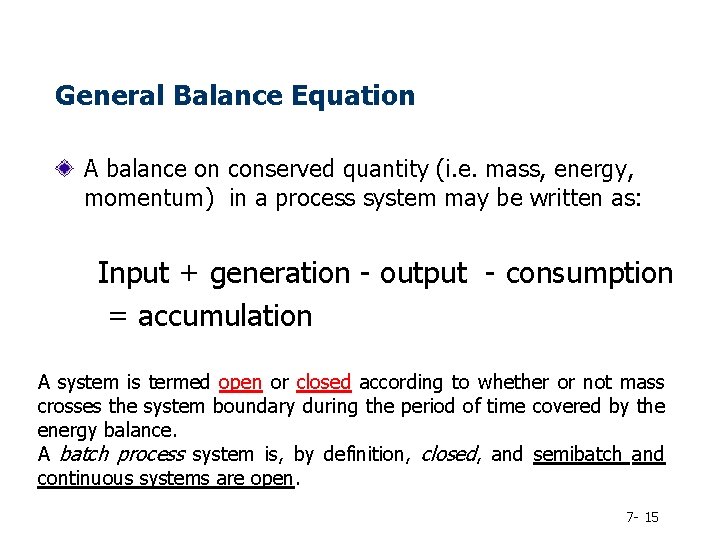
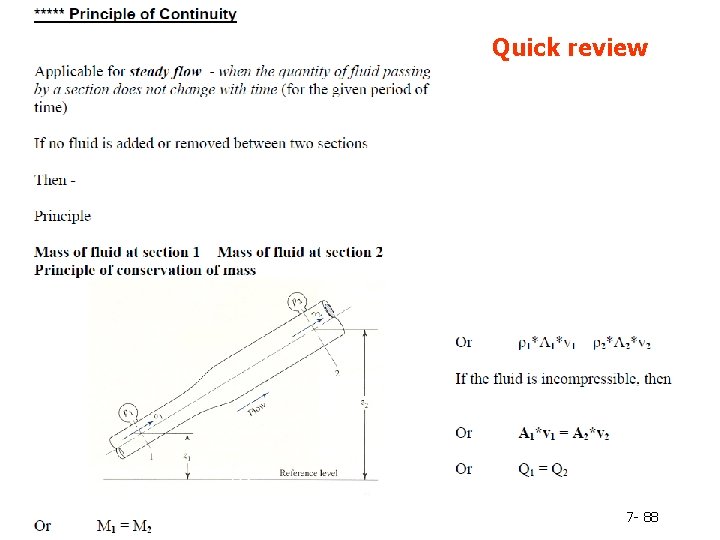
General Balance Equation A balance on conserved quantity (i. e. mass, energy, momentum) in a process system may be written as: Input + generation - output - consumption = accumulation A system is termed open or closed according to whether or not mass crosses the system boundary during the period of time covered by the energy balance. A batch process system is, by definition, closed, and semibatch and continuous systems are open. 7 - 15

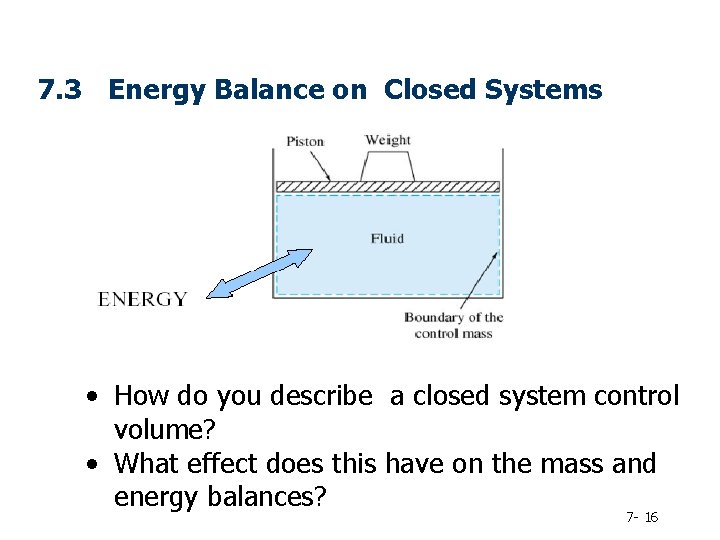
7. 3 Energy Balance on Closed Systems • How do you describe a closed system control volume? • What effect does this have on the mass and energy balances? 7 - 16

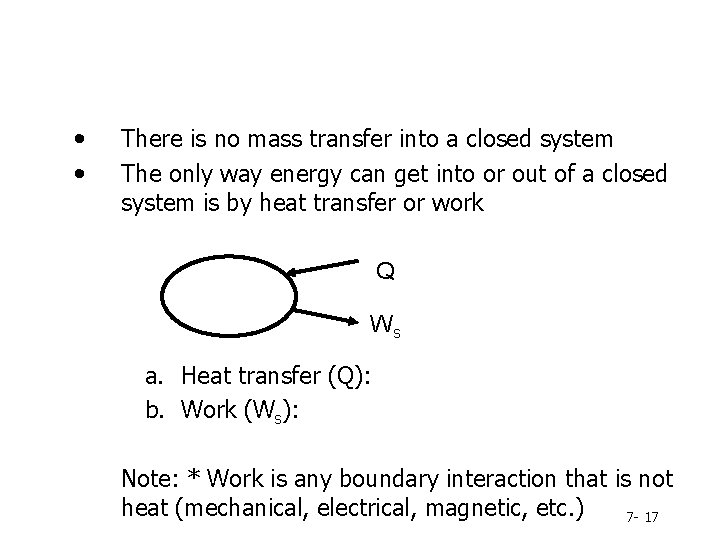
• • There is no mass transfer into a closed system The only way energy can get into or out of a closed system is by heat transfer or work Q Ws a. Heat transfer (Q): b. Work (Ws): Note: * Work is any boundary interaction that is not heat (mechanical, electrical, magnetic, etc. ) 7 - 17

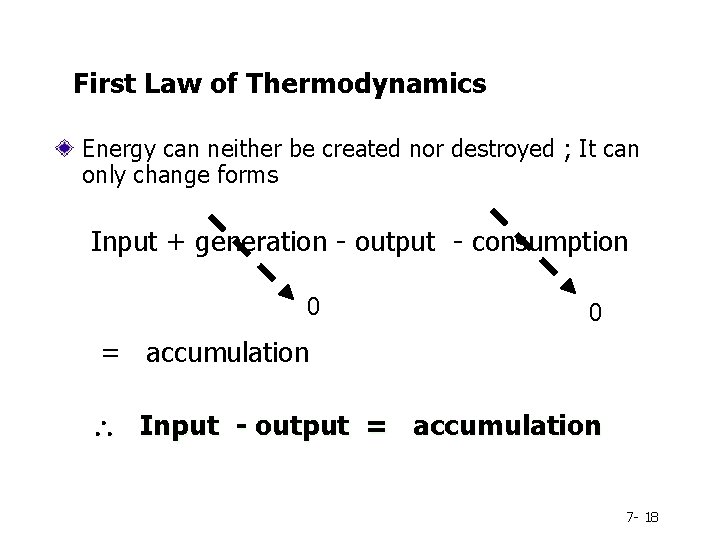
First Law of Thermodynamics Energy can neither be created nor destroyed ; It can only change forms Input + generation - output - consumption 0 0 = accumulation Input - output = accumulation 7 - 18

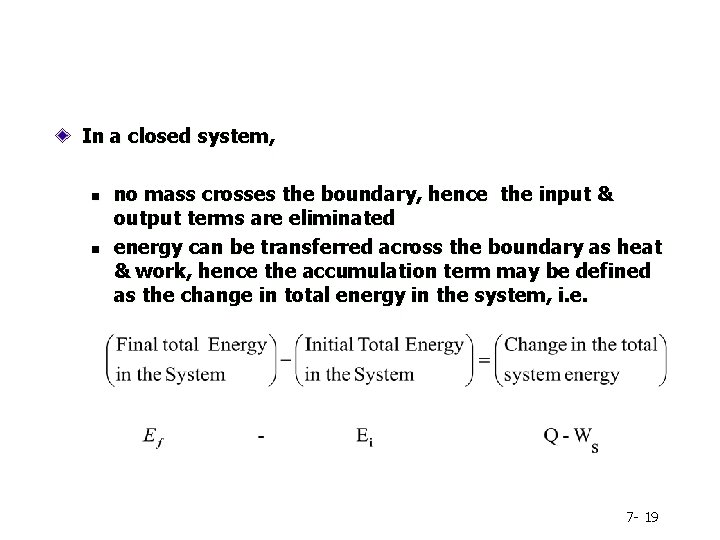
In a closed system, n n no mass crosses the boundary, hence the input & output terms are eliminated energy can be transferred across the boundary as heat & work, hence the accumulation term may be defined as the change in total energy in the system, i. e. 7 - 19

Q = heat transferred to the system Ws = work done by the system 7 - 20

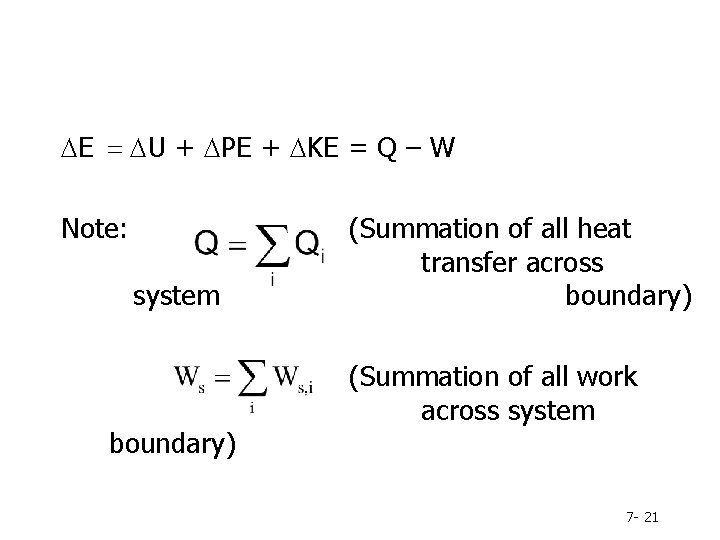
DE = DU + DPE + DKE = Q – W Note: system boundary) (Summation of all heat transfer across boundary) (Summation of all work across system 7 - 21

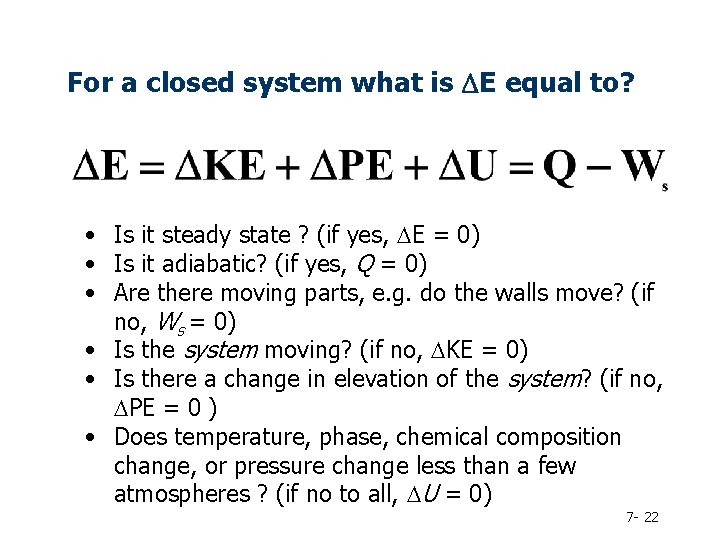
For a closed system what is DE equal to? • Is it steady state ? (if yes, DE = 0) • Is it adiabatic? (if yes, Q = 0) • Are there moving parts, e. g. do the walls move? (if no, Ws = 0) • Is the system moving? (if no, DKE = 0) • Is there a change in elevation of the system? (if no, DPE = 0 ) • Does temperature, phase, chemical composition change, or pressure change less than a few atmospheres ? (if no to all, DU = 0) 7 - 22

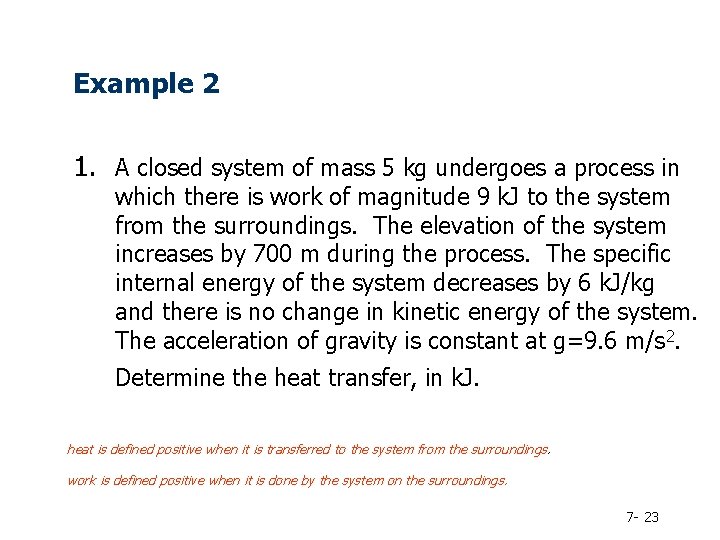
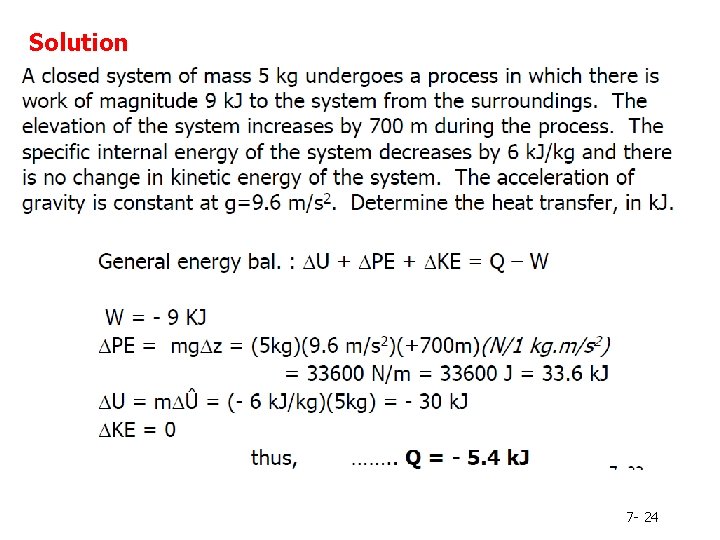
Example 2 1. A closed system of mass 5 kg undergoes a process in which there is work of magnitude 9 k. J to the system from the surroundings. The elevation of the system increases by 700 m during the process. The specific internal energy of the system decreases by 6 k. J/kg and there is no change in kinetic energy of the system. The acceleration of gravity is constant at g=9. 6 m/s 2. Determine the heat transfer, in k. J. heat is defined positive when it is transferred to the system from the surroundings. work is defined positive when it is done by the system on the surroundings. 7 - 23

Solution 7 - 24

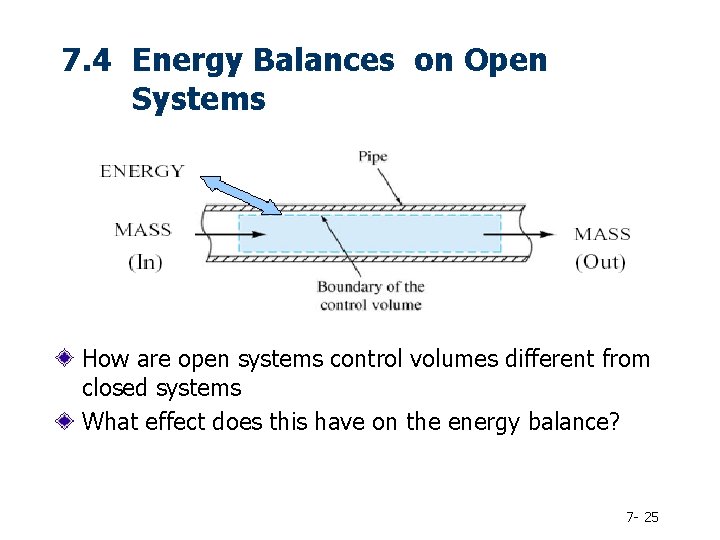
7. 4 Energy Balances on Open Systems How are open systems control volumes different from closed systems What effect does this have on the energy balance? 7 - 25

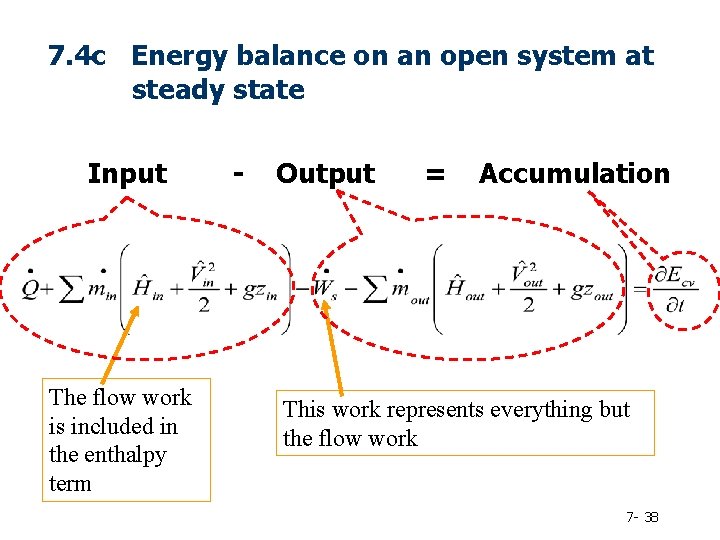
7. 4 ENERGY BALANCES ON OPEN SYSTEMS AT STEADY STATE 7. 4 a Flow Work and Shaft Work 7. 4 b Specific Properties and Enthalpy 7. 4 c The Steady-State Open-System Energy Balance 7 - 26

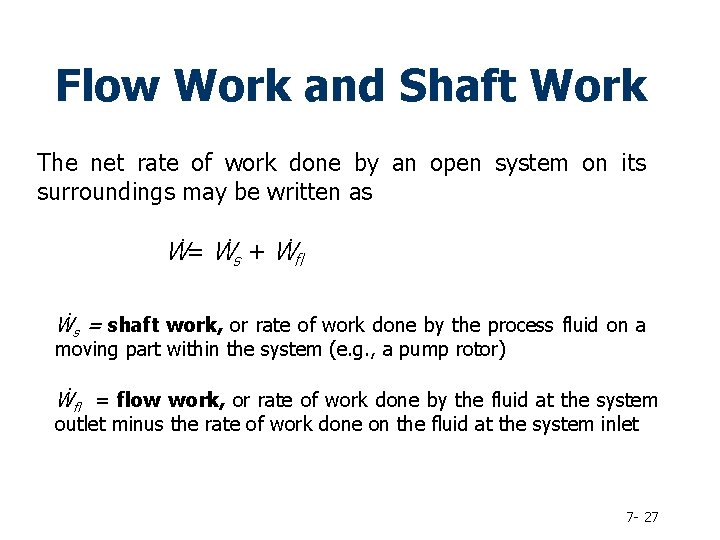
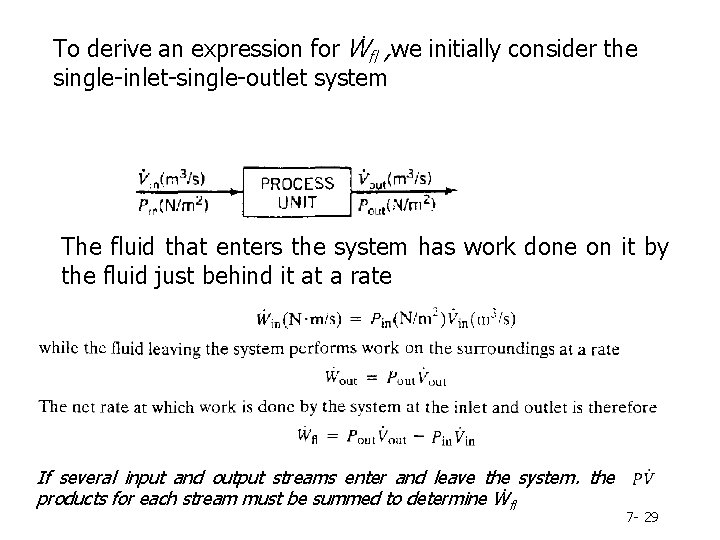
Flow Work and Shaft Work The net rate of work done by an open system on its surroundings may be written as Ẇ= Ẇs + Ẇfl Ẇs = shaft work, or rate of work done by the process fluid on a moving part within the system (e. g. , a pump rotor) Ẇfl = flow work, or rate of work done by the fluid at the system outlet minus the rate of work done on the fluid at the system inlet 7 - 27

heat is defined positive when it is transferred to the system from the surroundings. work is defined positive when it is done by the system on the surroundings. 7 - 28

To derive an expression for Ẇfl , we initially consider the single-inlet-single-outlet system The fluid that enters the system has work done on it by the fluid just behind it at a rate If several input and output streams enter and leave the system. the products for each stream must be summed to determine Ẇfl 7 - 29

7. 4 b Specific Properties and Enthalpy The properties of a process material are either extensive (proportional to the quantity of the material) or intensive (independent of the quantity) Mass, number of moles, and volume (or mass flow rate, molar flow rate and volumetric flow rate for a continuous stream), and kinetic energy, potential energy, and internal energy (or the rates of transport of these quantities by a continuous stream) are extensive properties Intensive: temperature, pressure, and density 7 - 30

We will use the symbol ᶺ to denote a specific property A property that occurs in the energy balance equation for open systems is the specific enthalpy, defined as 7 - 31

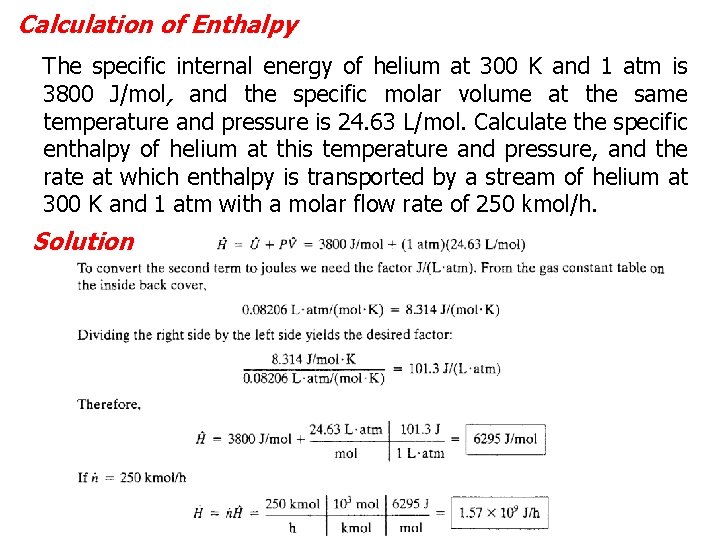
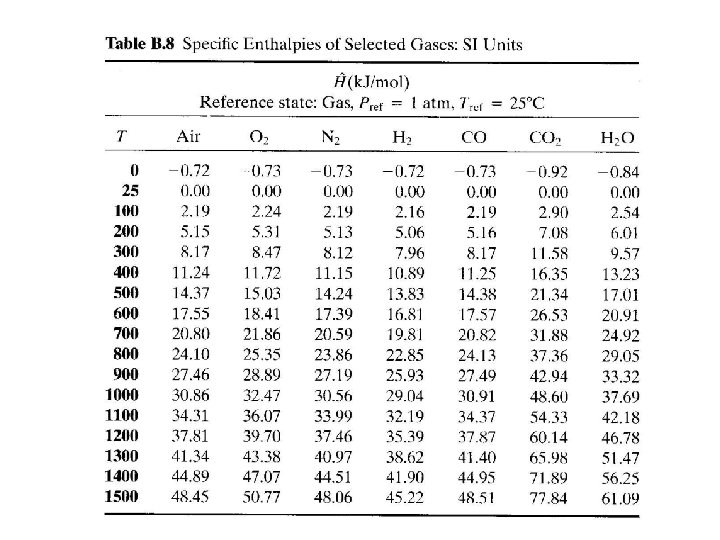
Calculation of Enthalpy The specific internal energy of helium at 300 K and 1 atm is 3800 J/mol, and the specific molar volume at the same temperature and pressure is 24. 63 L/mol. Calculate the specific enthalpy of helium at this temperature and pressure, and the rate at which enthalpy is transported by a stream of helium at 300 K and 1 atm with a molar flow rate of 250 kmol/h. Solution 7 - 32

TEST YOURSELF The specific internal energy of a fluid is 200 cal/g. 1. What is the internal energy of 30 g of this fluid? 2. If the fluid leaves a system at a flow rate of 5 g/min, at what rate does it transport internal energy out of the system? 3. What would you need to know to calculate the specific enthalpy of this fluid? 7 - 33

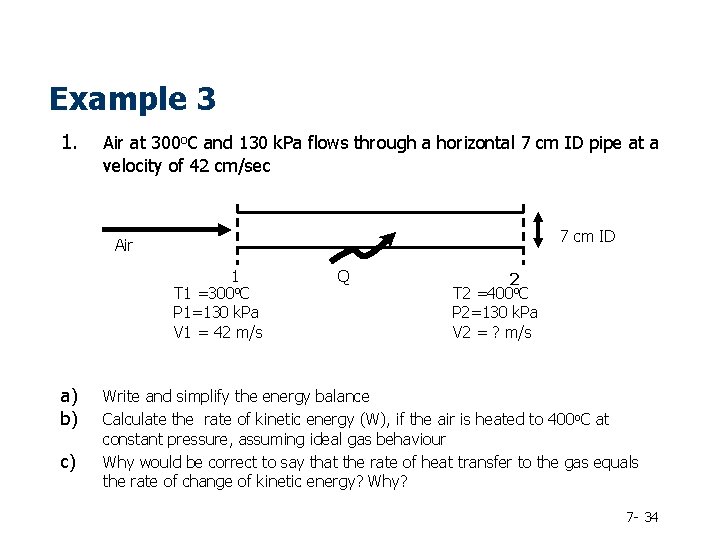
Example 3 1. Air at 300 o. C and 130 k. Pa flows through a horizontal 7 cm ID pipe at a velocity of 42 cm/sec 7 cm ID Air 1 T 1 =300 o. C P 1=130 k. Pa V 1 = 42 m/s a) b) c) Q 2 T 2 =400 o. C P 2=130 k. Pa V 2 = ? m/s Write and simplify the energy balance Calculate the rate of kinetic energy (W), if the air is heated to 400 o. C at constant pressure, assuming ideal gas behaviour Why would be correct to say that the rate of heat transfer to the gas equals the rate of change of kinetic energy? Why? 7 - 34

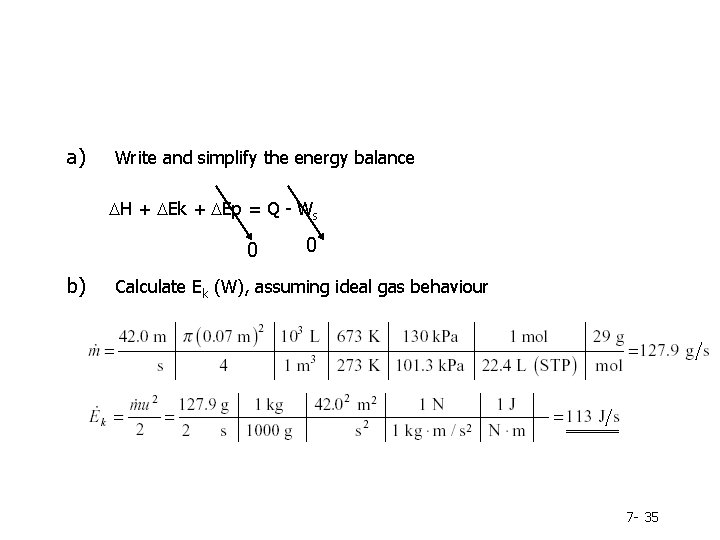
a) Write and simplify the energy balance DH + DEk + DEp = Q - Ws 0 b) 0 Calculate Ek (W), assuming ideal gas behaviour 7 - 35

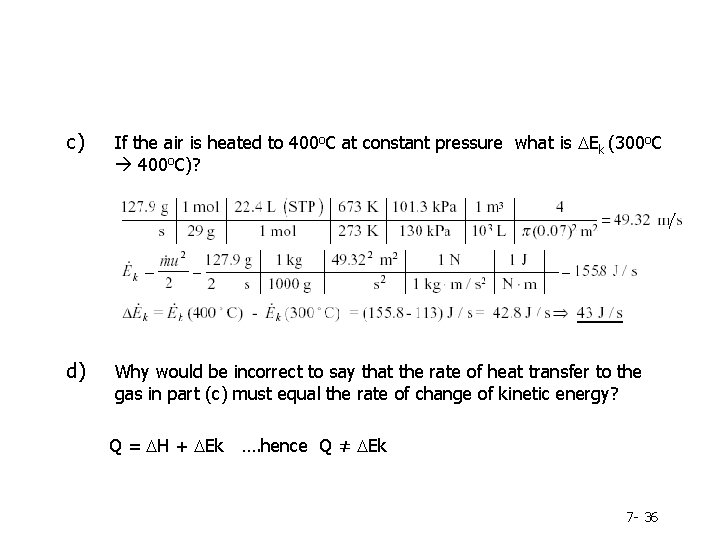
c) If the air is heated to 400 o. C at constant pressure what is DEk (300 o. C 400 o. C)? d) Why would be incorrect to say that the rate of heat transfer to the gas in part (c) must equal the rate of change of kinetic energy? Q = DH + DEk …. hence Q ≠ DEk 7 - 36

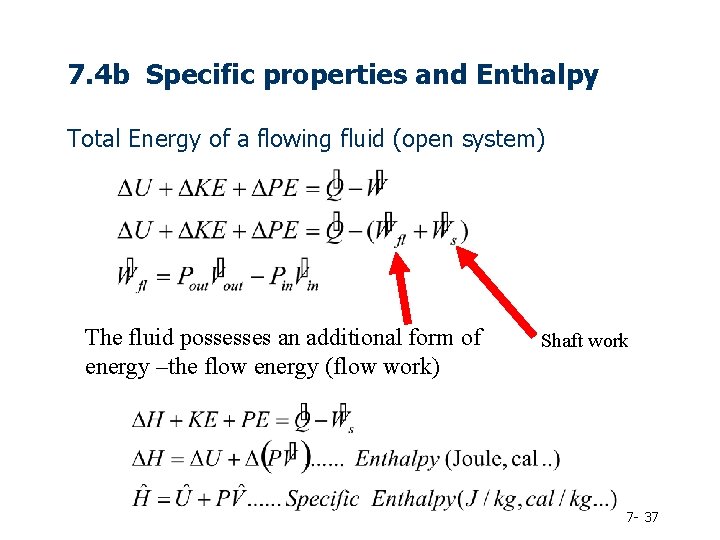
7. 4 b Specific properties and Enthalpy Total Energy of a flowing fluid (open system) The fluid possesses an additional form of energy –the flow energy (flow work) Shaft work 7 - 37

7. 4 c Energy balance on an open system at steady state Input The flow work is included in the enthalpy term - Output = Accumulation This work represents everything but the flow work 7 - 38

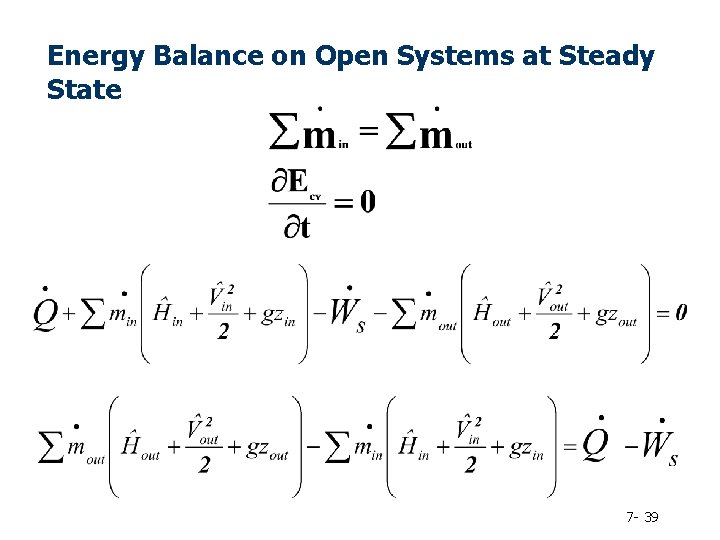
Energy Balance on Open Systems at Steady State 7 - 39

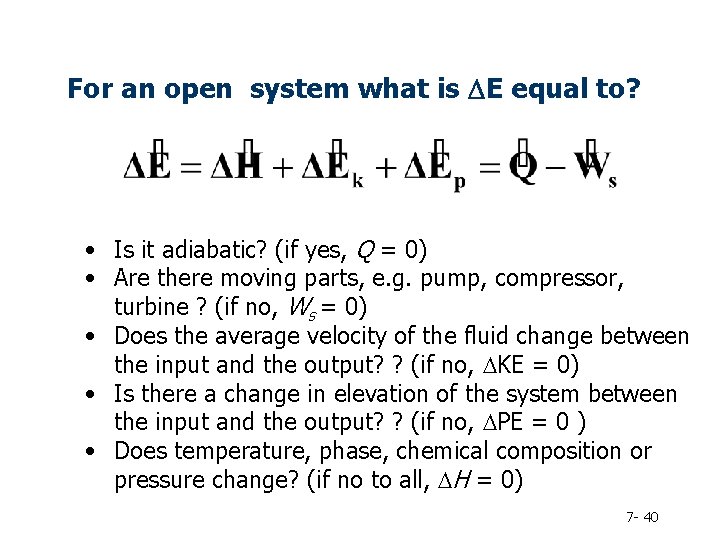
For an open system what is DE equal to? • Is it adiabatic? (if yes, Q = 0) • Are there moving parts, e. g. pump, compressor, turbine ? (if no, Ws = 0) • Does the average velocity of the fluid change between the input and the output? ? (if no, DKE = 0) • Is there a change in elevation of the system between the input and the output? ? (if no, DPE = 0 ) • Does temperature, phase, chemical composition or pressure change? (if no to all, DH = 0) 7 - 40

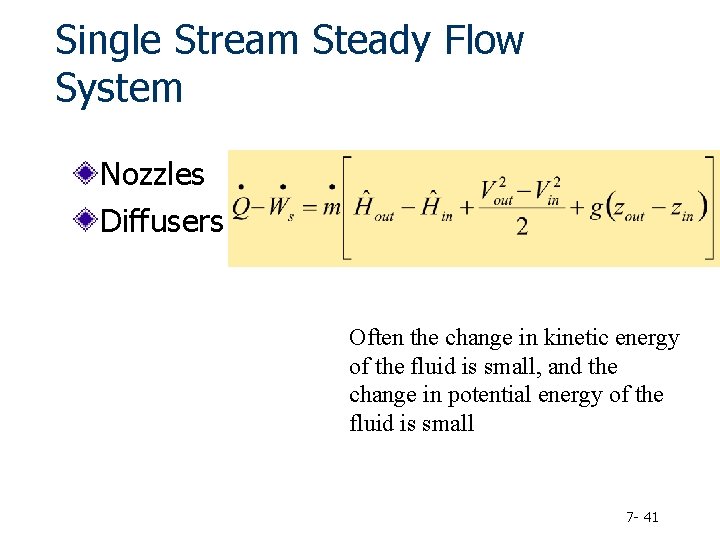
Single Stream Steady Flow System Nozzles Diffusers Often the change in kinetic energy of the fluid is small, and the change in potential energy of the fluid is small 7 - 41

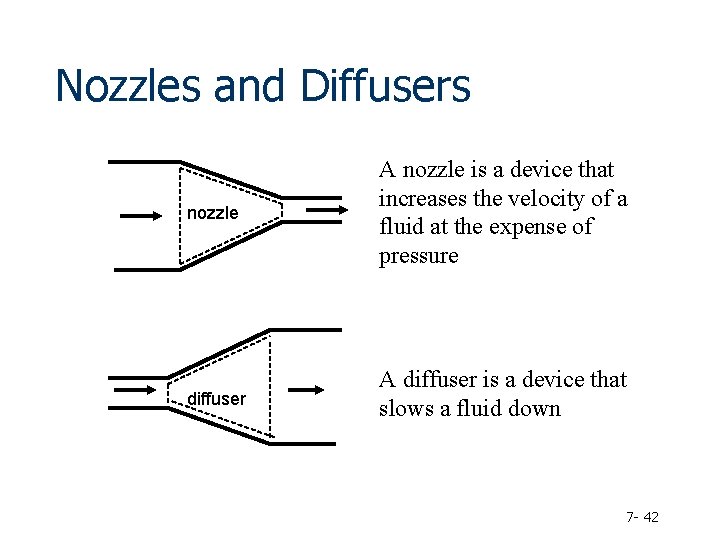
Nozzles and Diffusers nozzle A nozzle is a device that increases the velocity of a fluid at the expense of pressure diffuser A diffuser is a device that slows a fluid down 7 - 42

Is there work in this system? NO Is there heat transfer? Usually it can be ignored Does the fluid change elevation? NO enthalpy is converted into kinetic energy 7 - 43

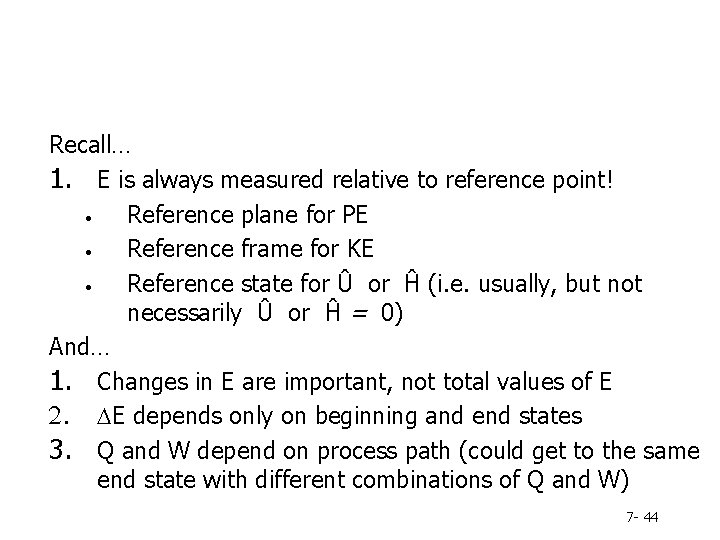
Recall… 1. E is always measured relative to reference point! • Reference plane for PE • Reference frame for KE • Reference state for Û or Ĥ (i. e. usually, but not necessarily Û or Ĥ = 0) And… 1. Changes in E are important, not total values of E 2. DE depends only on beginning and end states 3. Q and W depend on process path (could get to the same end state with different combinations of Q and W) 7 - 44

7. 5 Tables of Thermodynamic Data Recall …. Thermodynamics Entropy is, remarkably, a function of state. Entropy is an extensive property, but it is often given as an intensive property of specific entropy as entropy per unit mass or entropy per mole. 7 - 45

7 - 46

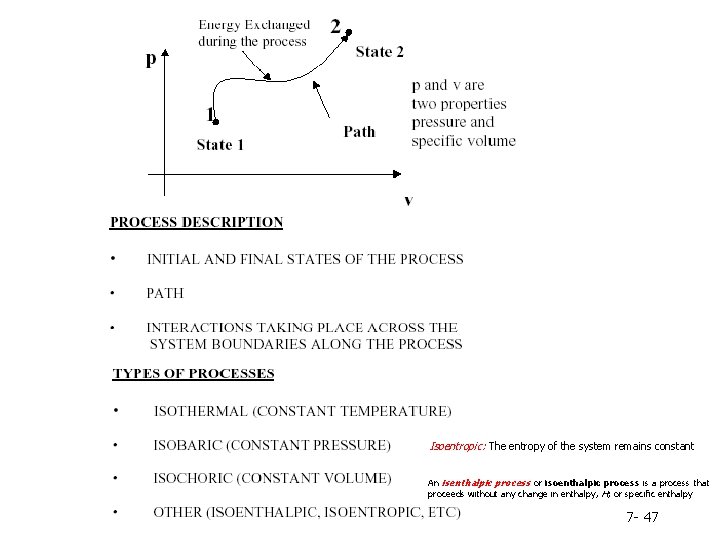
Isoentropic: The entropy of the system remains constant An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy 7 - 47

7. 5 a Reference states and state properties • State property – a property of a system component whose value depends only on the state of the system (i. e. temperature, pressure, phase and composition)… e. g. internal energy (U) and hence, enthalpy (H) • It is impossible to measure the absolute value of state property … but can estimate the change in specific value of U (i. e DÛ) or H (i. e DĤ) corresponding to a specified change of state (i. e. temperature, pressure, phase and composition) • Reference state - specified state (i. e. temperature, pressure or state of aggregation) assigned to measure relative changes in Û or Ĥ … thus, the value of Û or Ĥ of a certain material at a specified state (T, P or phase) is relative to the value of Û or Ĥ of the same material at other specified state (T, P or phase) 7 - 48

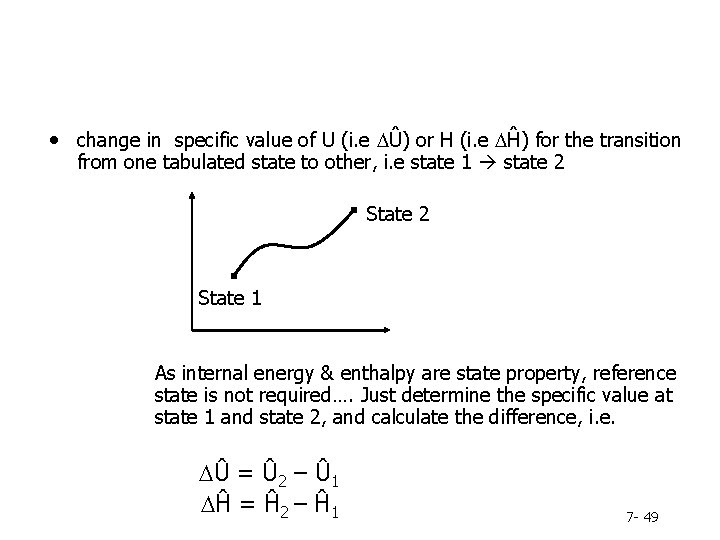
• change in specific value of U (i. e DÛ) or H (i. e DĤ) for the transition from one tabulated state to other, i. e state 1 state 2 State 1 As internal energy & enthalpy are state property, reference state is not required…. Just determine the specific value at state 1 and state 2, and calculate the difference, i. e. DÛ = Û 2 – Û 1 DĤ = Ĥ 2 – Ĥ 1 7 - 49

7 - 50

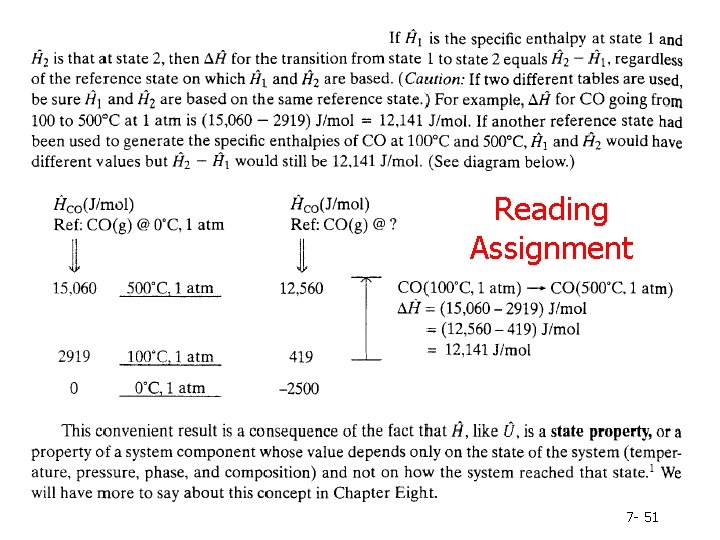
Reading Assignment 7 - 51

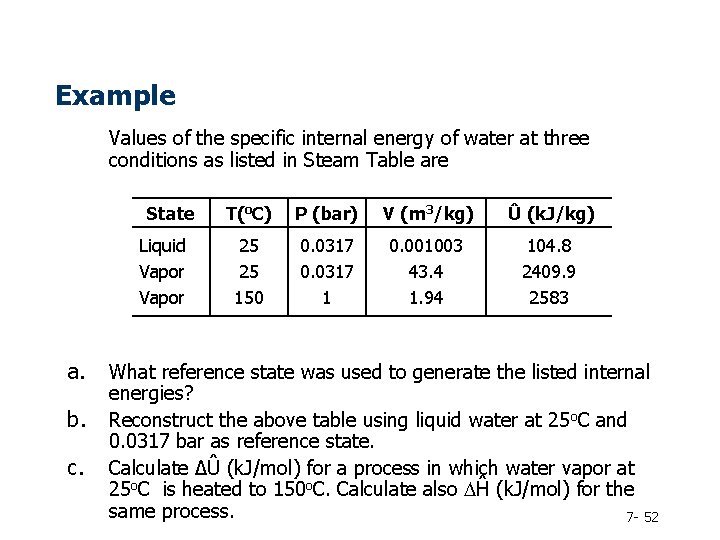
Example Values of the specific internal energy of water at three conditions as listed in Steam Table are State Liquid Vapor a. b. c. T(o. C) P (bar) V (m 3/kg) Û (k. J/kg) 25 25 150 0. 0317 1 0. 001003 43. 4 1. 94 104. 8 2409. 9 2583 What reference state was used to generate the listed internal energies? Reconstruct the above table using liquid water at 25 o. C and 0. 0317 bar as reference state. Calculate ∆Û (k. J/mol) for a process in which water vapor at 25 o. C is heated to 150 o. C. Calculate also DĤ (k. J/mol) for the same process. 7 - 52

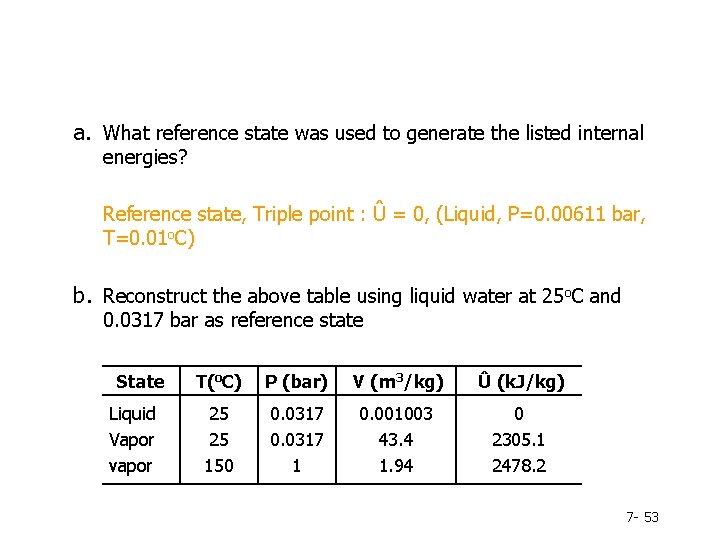
a. What reference state was used to generate the listed internal energies? Reference state, Triple point : Û = 0, (Liquid, P=0. 00611 bar, T=0. 01 o. C) b. Reconstruct the above table using liquid water at 25 o. C and 0. 0317 bar as reference state State Liquid Vapor vapor T(o. C) P (bar) V (m 3/kg) Û (k. J/kg) 25 25 150 0. 0317 1 0. 001003 43. 4 1. 94 0 2305. 1 2478. 2 7 - 53

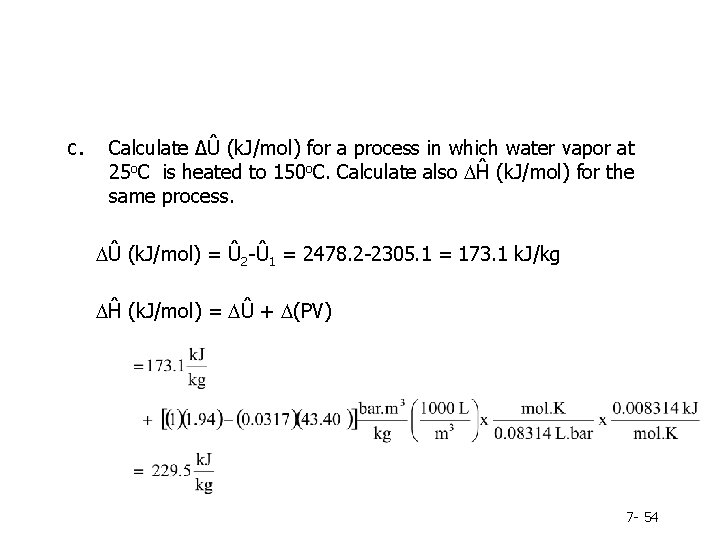
c. Calculate ∆Û (k. J/mol) for a process in which water vapor at 25 o. C is heated to 150 o. C. Calculate also DĤ (k. J/mol) for the same process. DÛ (k. J/mol) = Û 2 -Û 1 = 2478. 2 -2305. 1 = 173. 1 k. J/kg DĤ (k. J/mol) = DÛ + D(PV) 7 - 54

7 - 55

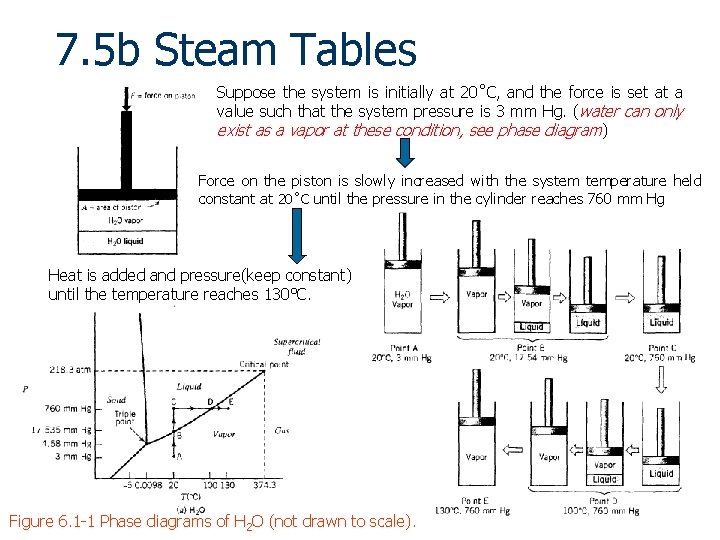
7. 5 b Steam Tables Suppose the system is initially at 20˚C, and the force is set at a value such that the system pressure is 3 mm Hg. (water can only exist as a vapor at these condition, see phase diagram) Force on the piston is slowly increased with the system temperature held constant at 20˚C until the pressure in the cylinder reaches 760 mm Hg Heat is added and pressure(keep constant) until the temperature reaches 130°C. Figure 6. 1 -1 Phase diagrams of H 2 O (not drawn to scale). 7 - 56

Several familiar terms may be defined with reference to the phase diagram. 1. If T and P correspond to a point on the vapor-liquid equilibrium curve for a substance, P is the vapor pressure of the substance at temperature T, and T is the boiling point (more precisely, the boiling point temperature) of the substance at pressure P. 2. The boiling point of a substance at P = 1 atm is the normal boiling point of that substance. 3. If (T, P) falls on the solid-liquid equilibrium curve, then T is the melting point or freezing point at pressure P. 7 - 57

4. If (T, P) falls on the solid-vapor equilibrium curve, then P is the vapor pressure of the solid at temperature T, and T is the sublimation point at pressure P. 5. The point (T, P) at which solid, liquid, and vapor phases can all coexist is called the triple point of the substance. 6. The vapor-liquid equilibrium curve terminates at the critical temperature and critical pressure (Tc and Pc). Above and to the right of the critical point, two separate phases never coexist. 7 - 58

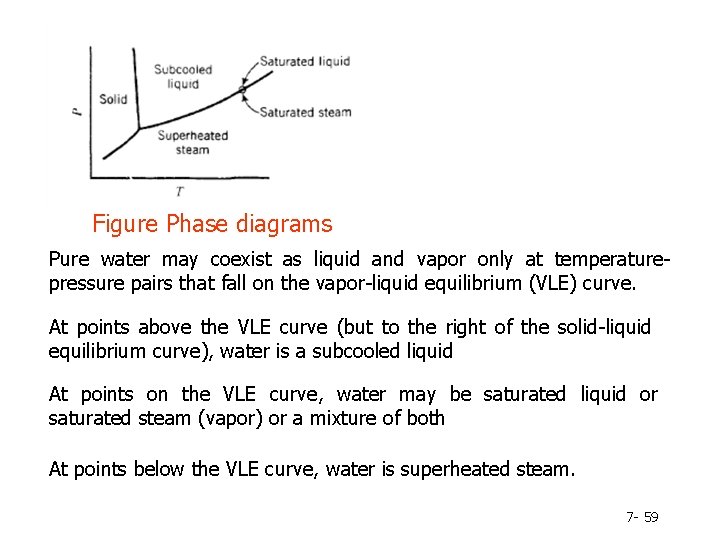
Figure Phase diagrams Pure water may coexist as liquid and vapor only at temperaturepressure pairs that fall on the vapor-liquid equilibrium (VLE) curve. At points above the VLE curve (but to the right of the solid-liquid equilibrium curve), water is a subcooled liquid At points on the VLE curve, water may be saturated liquid or saturated steam (vapor) or a mixture of both At points below the VLE curve, water is superheated steam. 7 - 59

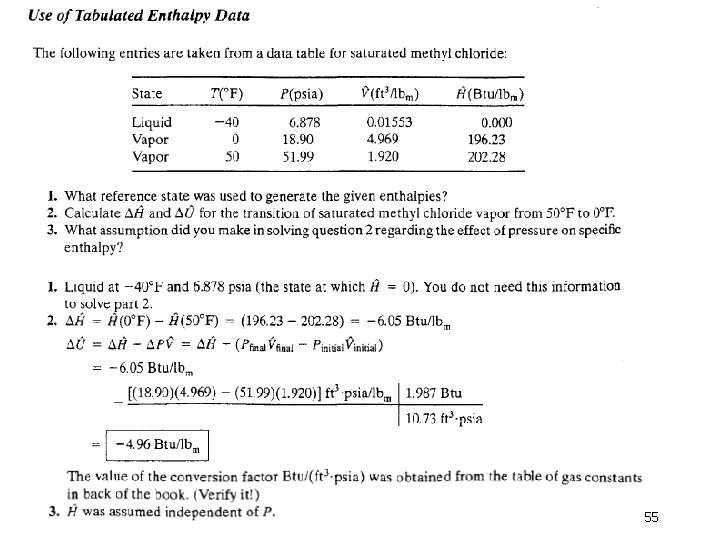
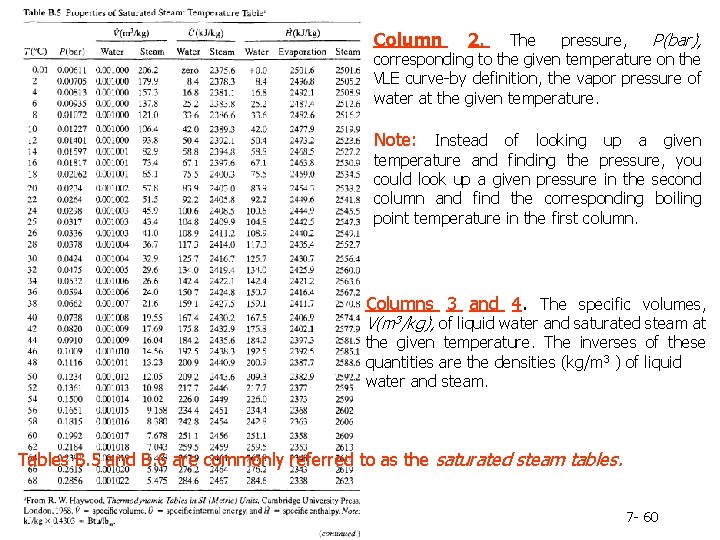
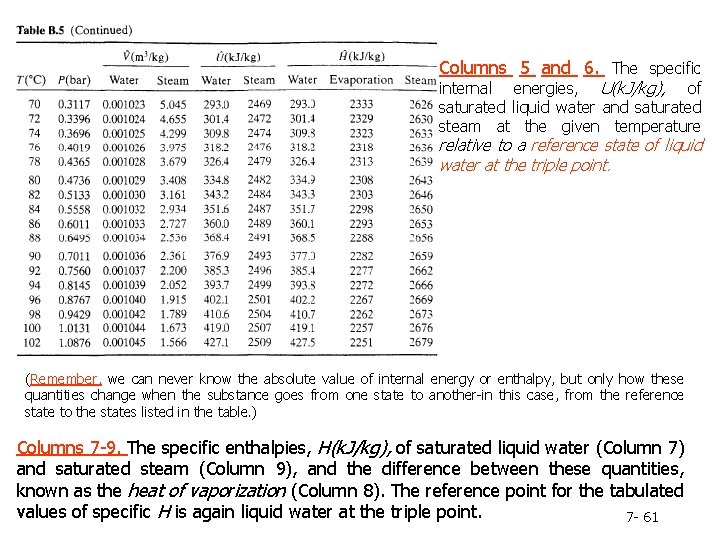
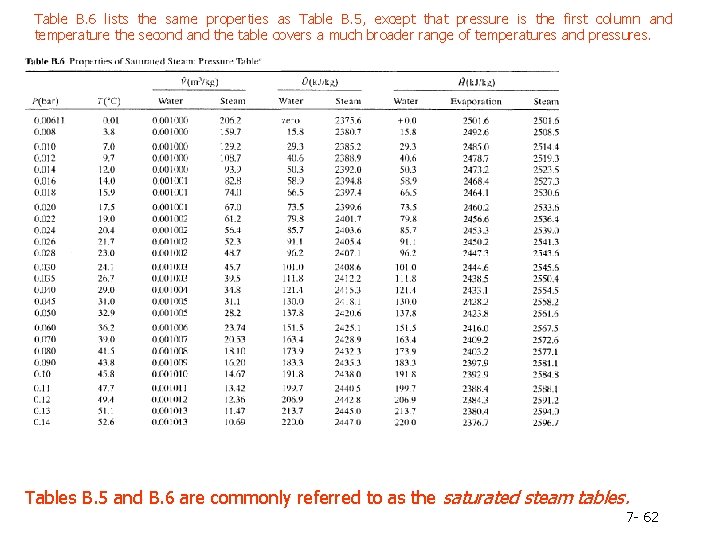
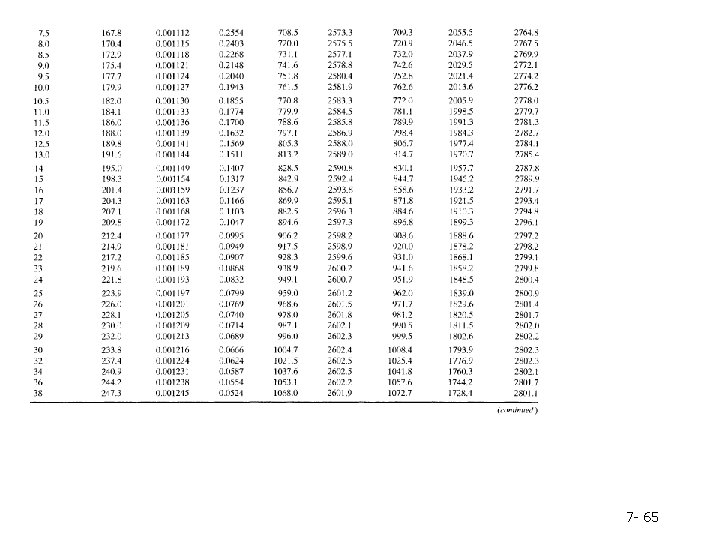
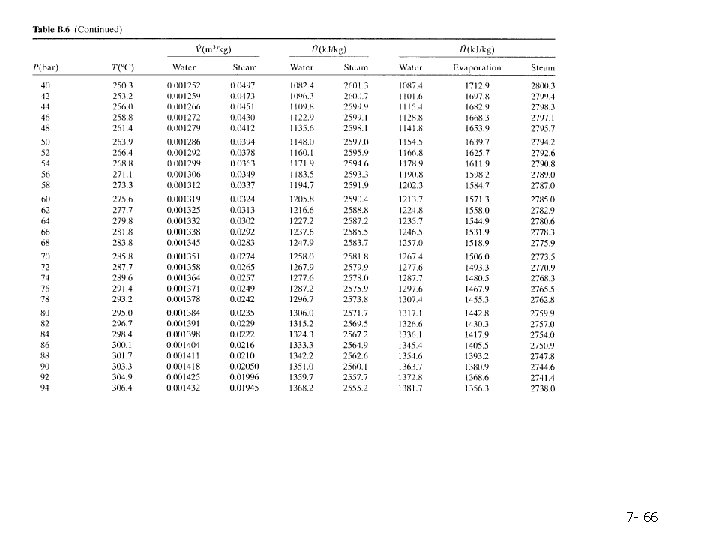
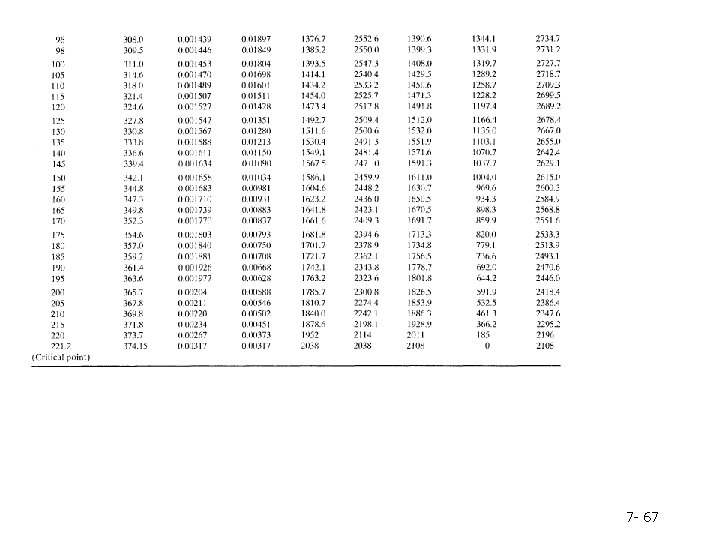
Column 2. The pressure, P(bar), corresponding to the given temperature on the VLE curve-by definition, the vapor pressure of water at the given temperature. Note: Instead of looking up a given temperature and finding the pressure, you could look up a given pressure in the second column and find the corresponding boiling point temperature in the first column. Columns 3 and 4. The specific volumes, V(m 3/kg), of liquid water and saturated steam at the given temperature. The inverses of these quantities are the densities (kg/m 3 ) of liquid water and steam. Tables B. 5 and B. 6 are commonly referred to as the saturated steam tables. 7 - 60

Columns 5 and 6. The specific internal energies, U(k. J/kg), of saturated liquid water and saturated steam at the given temperature relative to a reference state of liquid water at the triple point. (Remember, we can never know the absolute value of internal energy or enthalpy, but only how these quantities change when the substance goes from one state to another-in this case, from the reference state to the states listed in the table. ) Columns 7 -9. The specific enthalpies, H(k. J/kg), of saturated liquid water (Column 7) and saturated steam (Column 9), and the difference between these quantities, known as the heat of vaporization (Column 8). The reference point for the tabulated values of specific H is again liquid water at the triple point. 7 - 61

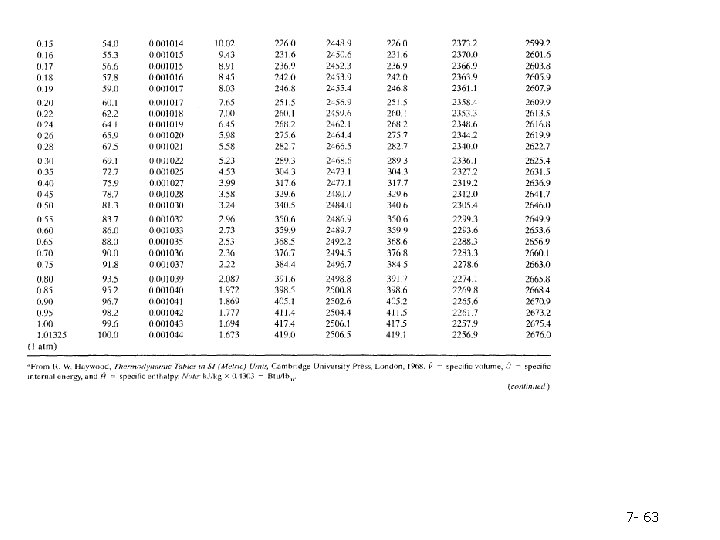
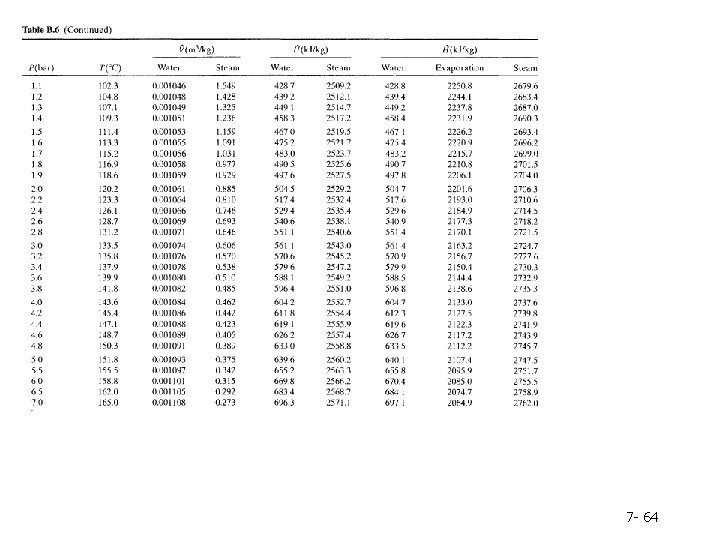
Table B. 6 lists the same properties as Table B. 5, except that pressure is the first column and temperature the second and the table covers a much broader range of temperatures and pressures. Tables B. 5 and B. 6 are commonly referred to as the saturated steam tables. 7 - 62

7 - 63

7 - 64

7 - 65

7 - 66

7 - 67

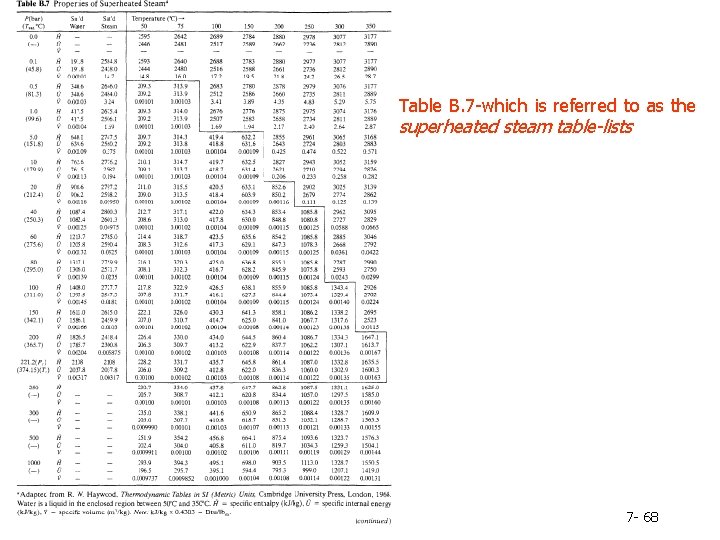
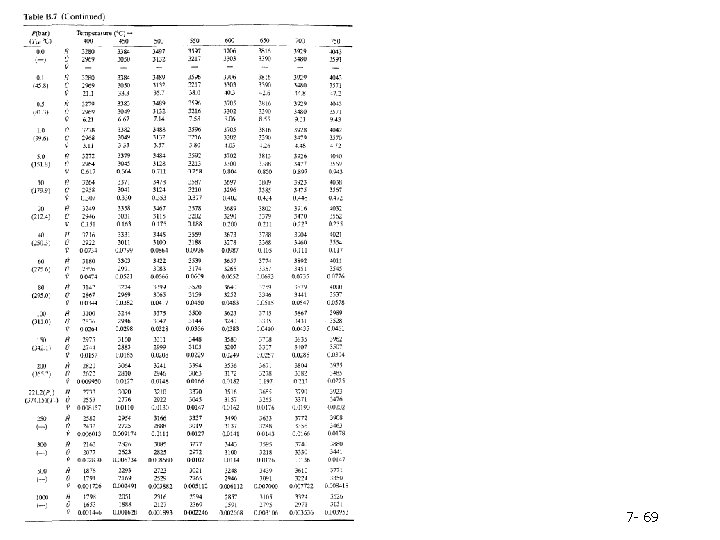
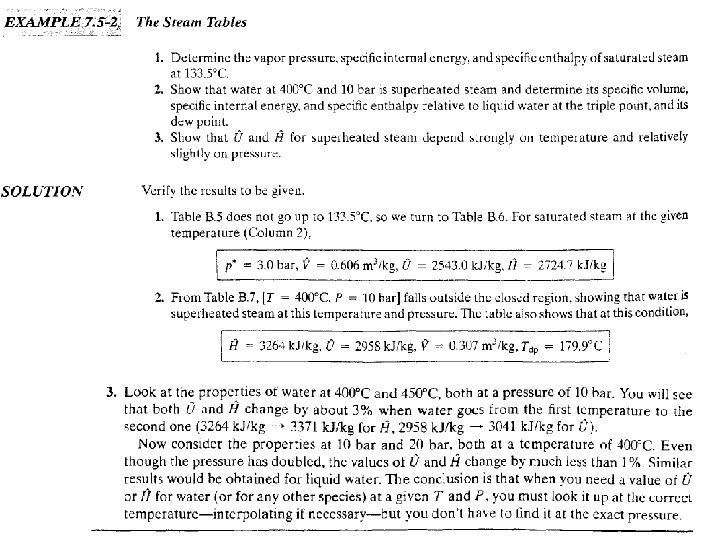
Table B. 7 -which is referred to as the superheated steam table-lists 7 - 68

7 - 69

7 - 70

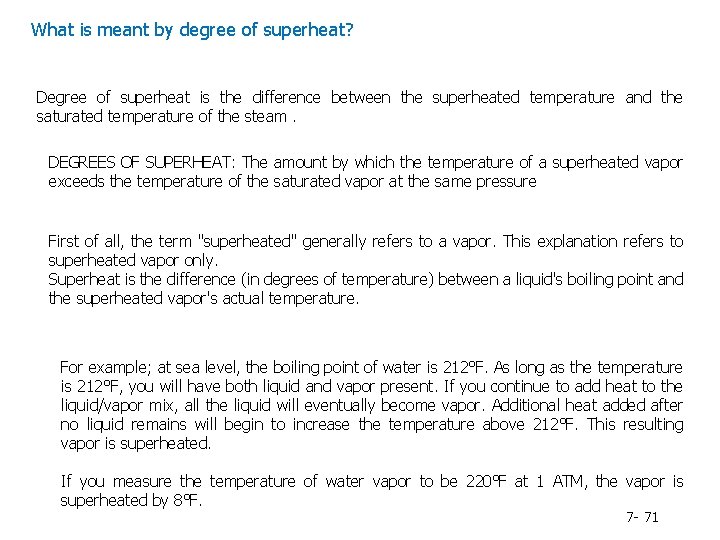
What is meant by degree of superheat? Degree of superheat is the difference between the superheated temperature and the saturated temperature of the steam. DEGREES OF SUPERHEAT: The amount by which the temperature of a superheated vapor exceeds the temperature of the saturated vapor at the same pressure First of all, the term "superheated" generally refers to a vapor. This explanation refers to superheated vapor only. Superheat is the difference (in degrees of temperature) between a liquid's boiling point and the superheated vapor's actual temperature. For example; at sea level, the boiling point of water is 212°F. As long as the temperature is 212°F, you will have both liquid and vapor present. If you continue to add heat to the liquid/vapor mix, all the liquid will eventually become vapor. Additional heat added after no liquid remains will begin to increase the temperature above 212°F. This resulting vapor is superheated. If you measure the temperature of water vapor to be 220°F at 1 ATM, the vapor is superheated by 8°F. 7 - 71

Superheated steam, as already stated, is steam the temperature of which exceeds that of saturated steam at the same pressure. It is produced by the addition of heat to saturated steam which has been removed from contact with the water from which it was generated 7 - 72

7. 6 ENERGY BALANCE PROCEDURES A properly drawn and labeled flowchart is essential for the efficient solution of energy balance problems. When labeling the flowchart, be sure to include all of the information you need to determine the specific enthalpy of each stream component including known temperatures and pressures. In addition, show states of aggregation of process materials when they are not obvious: do not simply write H 2 O, for example, but rather H 2 O(s), H 2 O(l), or H 2 O(v), according to whether water is present as a solid, a liquid, or a vapor. 7 - 73

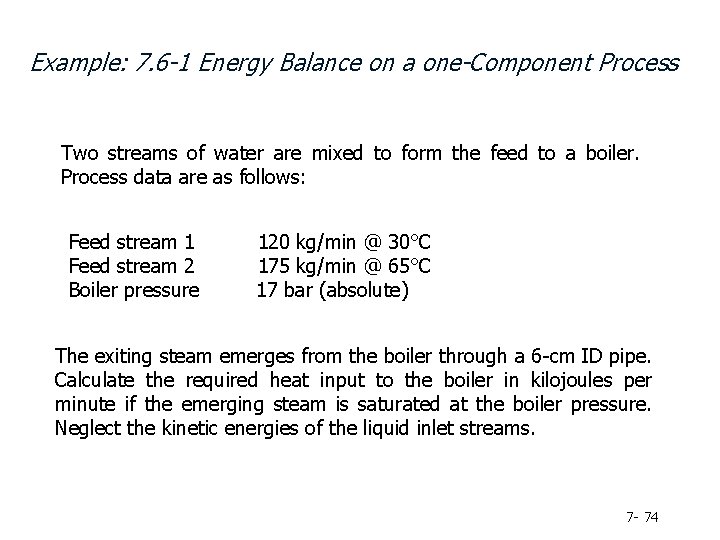
Example: 7. 6 -1 Energy Balance on a one-Component Process Two streams of water are mixed to form the feed to a boiler. Process data are as follows: Feed stream 1 Feed stream 2 Boiler pressure 120 kg/min @ 30°C 175 kg/min @ 65°C 17 bar (absolute) The exiting steam emerges from the boiler through a 6 -cm ID pipe. Calculate the required heat input to the boiler in kilojoules per minute if the emerging steam is saturated at the boiler pressure. Neglect the kinetic energies of the liquid inlet streams. 7 - 74

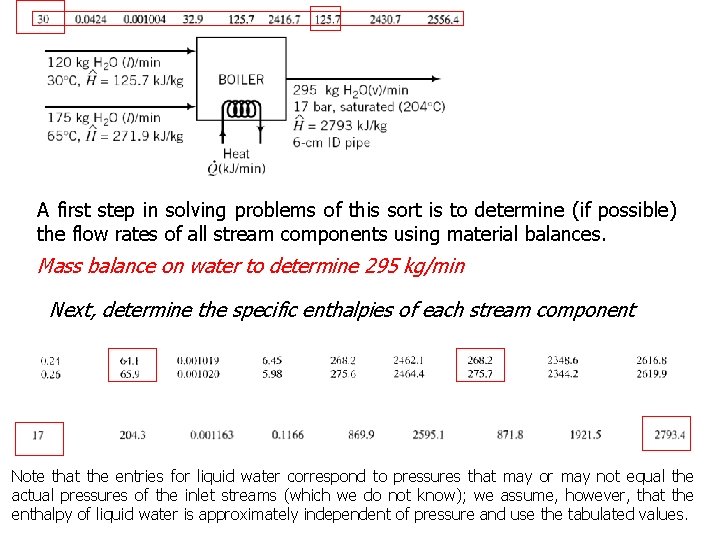
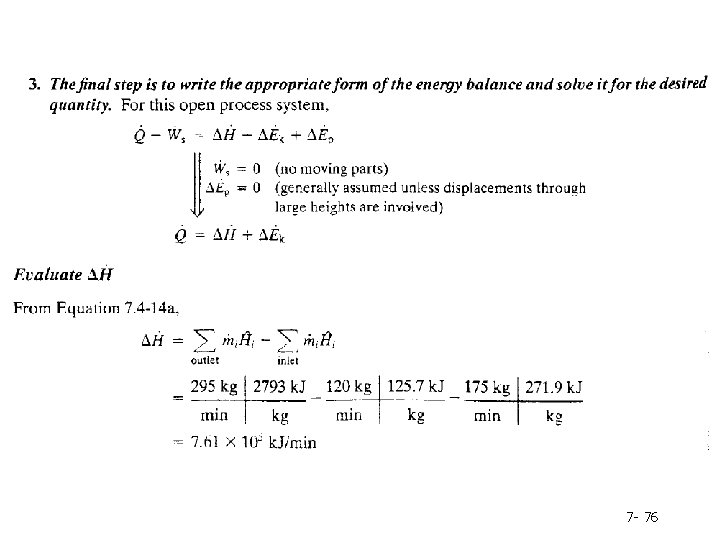
A first step in solving problems of this sort is to determine (if possible) the flow rates of all stream components using material balances. Mass balance on water to determine 295 kg/min Next, determine the specific enthalpies of each stream component Note that the entries for liquid water correspond to pressures that may or may not equal the actual pressures of the inlet streams (which we do not know); we assume, however, that the enthalpy of liquid water is approximately independent of pressure and use the tabulated values.

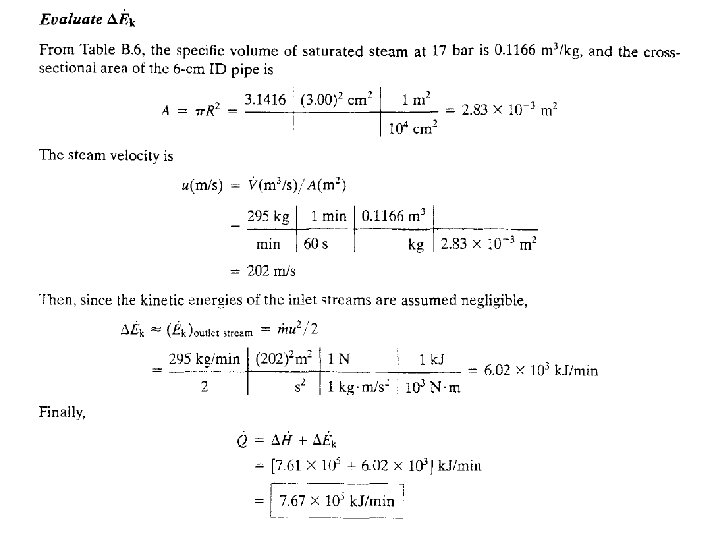
7 - 76

7 - 77

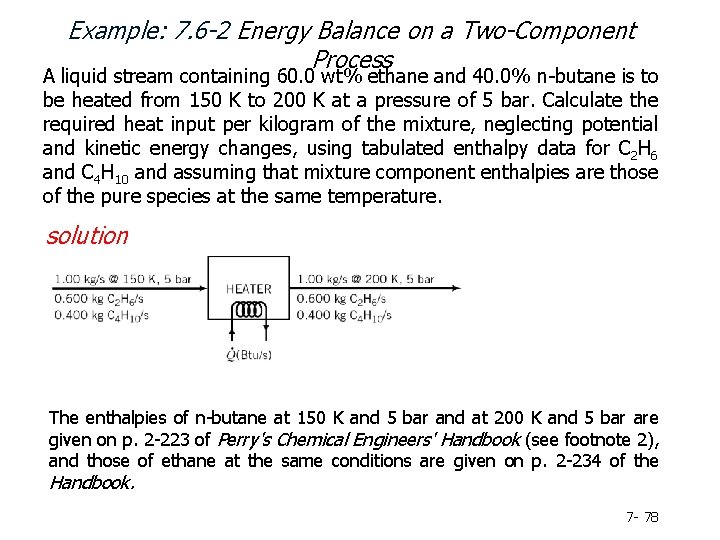
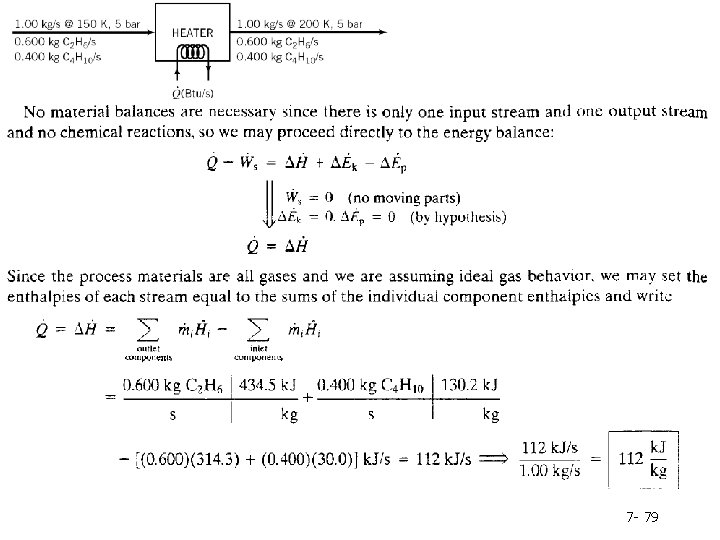
Example: 7. 6 -2 Energy Balance on a Two-Component Process A liquid stream containing 60. 0 wt% ethane and 40. 0% n-butane is to be heated from 150 K to 200 K at a pressure of 5 bar. Calculate the required heat input per kilogram of the mixture, neglecting potential and kinetic energy changes, using tabulated enthalpy data for C 2 H 6 and C 4 H 10 and assuming that mixture component enthalpies are those of the pure species at the same temperature. solution The enthalpies of n-butane at 150 K and 5 bar and at 200 K and 5 bar are given on p. 2 -223 of Perry's Chemical Engineers' Handbook (see footnote 2), and those of ethane at the same conditions are given on p. 2 -234 of the Handbook. 7 - 78

7 - 79

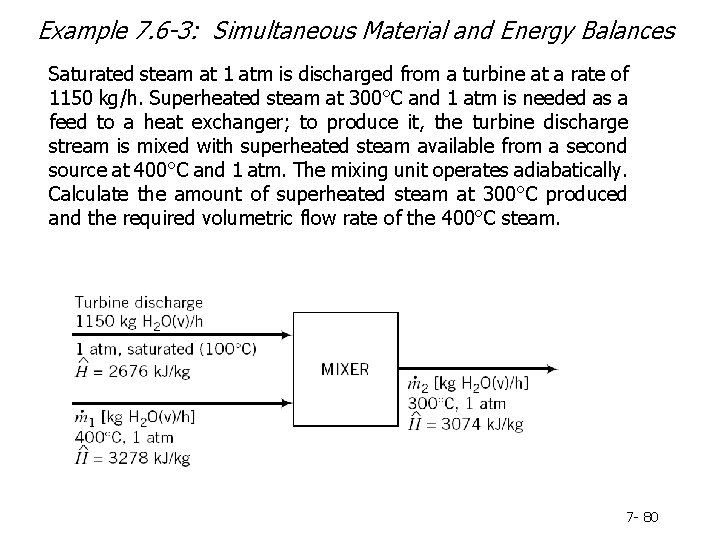
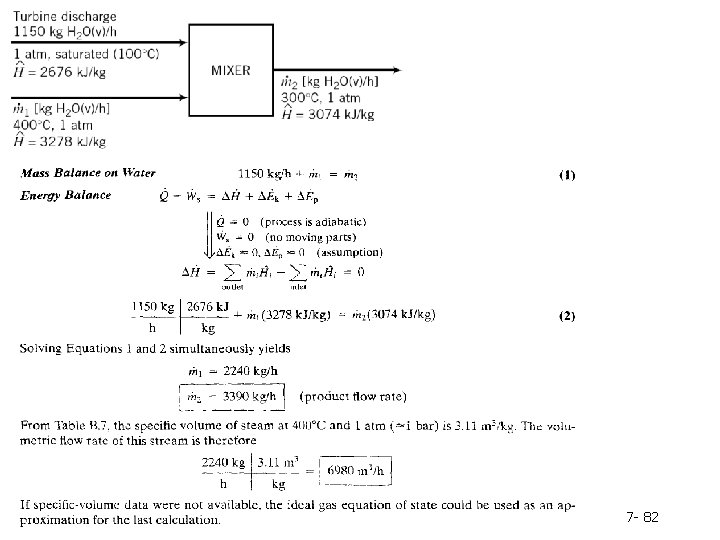
Example 7. 6 -3: Simultaneous Material and Energy Balances Saturated steam at 1 atm is discharged from a turbine at a rate of 1150 kg/h. Superheated steam at 300°C and 1 atm is needed as a feed to a heat exchanger; to produce it, the turbine discharge stream is mixed with superheated steam available from a second source at 400°C and 1 atm. The mixing unit operates adiabatically. Calculate the amount of superheated steam at 300°C produced and the required volumetric flow rate of the 400°C steam. 7 - 80

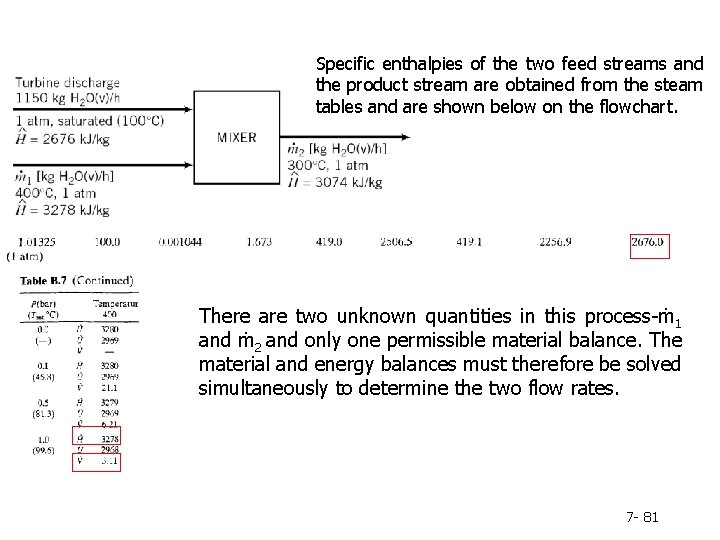
Specific enthalpies of the two feed streams and the product stream are obtained from the steam tables and are shown below on the flowchart. There are two unknown quantities in this process-ṁ1 and ṁ2 and only one permissible material balance. The material and energy balances must therefore be solved simultaneously to determine the two flow rates. 7 - 81

7 - 82

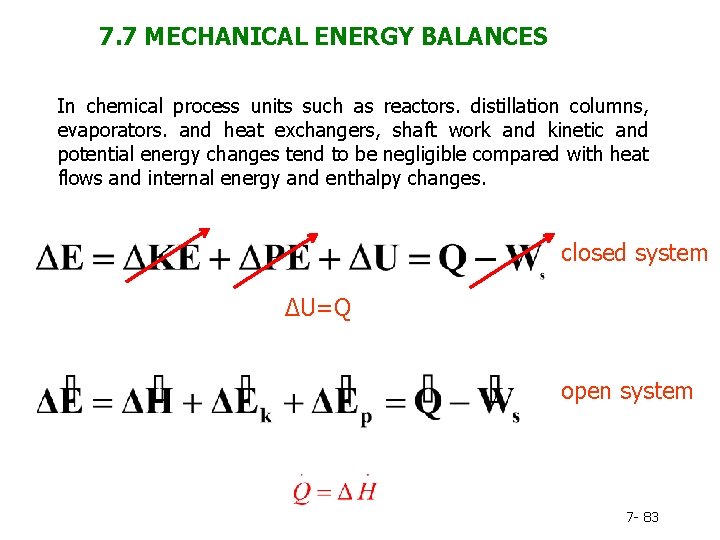
7. 7 MECHANICAL ENERGY BALANCES In chemical process units such as reactors. distillation columns, evaporators. and heat exchangers, shaft work and kinetic and potential energy changes tend to be negligible compared with heat flows and internal energy and enthalpy changes. closed system ΔU=Q open system 7 - 83

Another important class of operations is one for which the opposite is true: heat flows and internal energy changes are secondary in importance to kinetic and potential energy changes and shaft work. Examples: Most of these operations involve the flow of fluids to, from, and between tanks, reservoirs, wells, and process units. Accounting for energy flows in such processes is most conveniently done with mechanical energy balances. 7 - 84

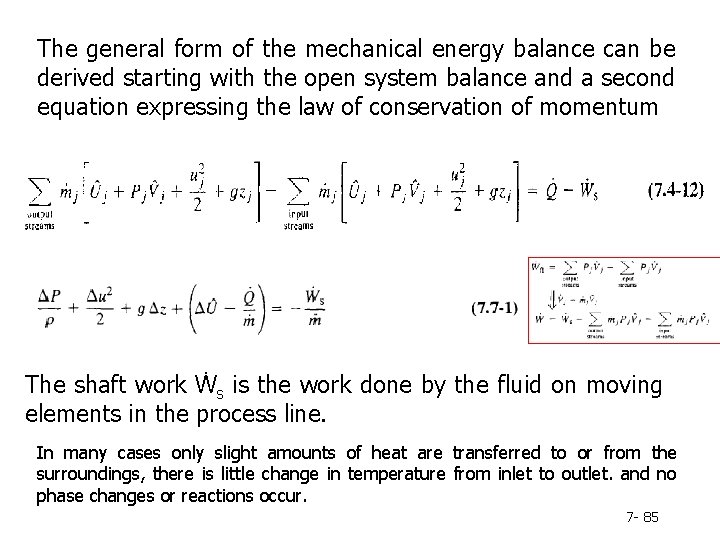
The general form of the mechanical energy balance can be derived starting with the open system balance and a second equation expressing the law of conservation of momentum The shaft work Ẇs is the work done by the fluid on moving elements in the process line. In many cases only slight amounts of heat are transferred to or from the surroundings, there is little change in temperature from inlet to outlet. and no phase changes or reactions occur. 7 - 85

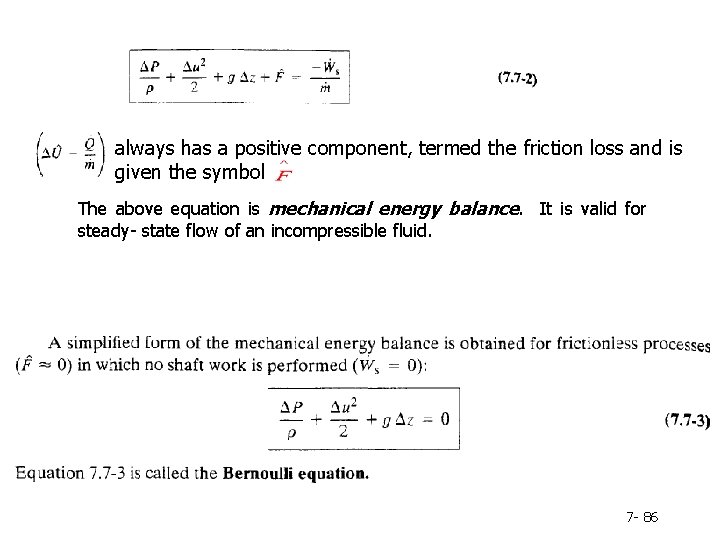
always has a positive component, termed the friction loss and is given the symbol The above equation is mechanical energy balance. It is valid for steady- state flow of an incompressible fluid. 7 - 86

heat is defined positive when it is transferred to the system from the surroundings. work is defined positive when it is done by the system on the surroundings. 7 - 87

Quick review 7 - 88

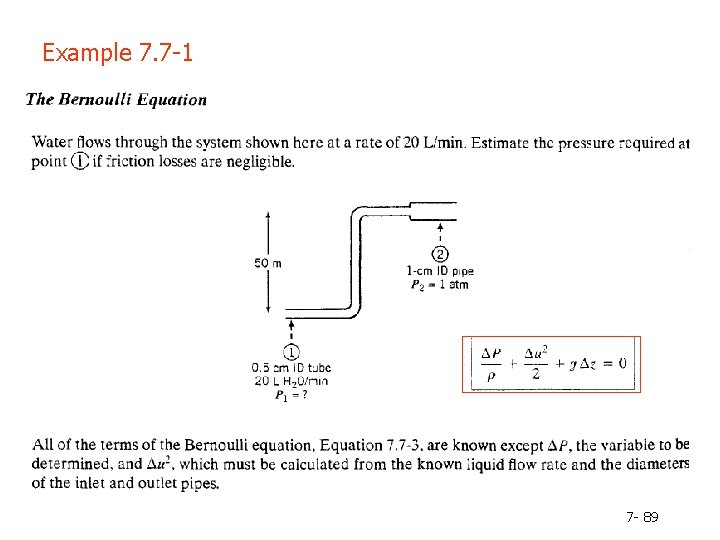
Example 7. 7 -1 7 - 89

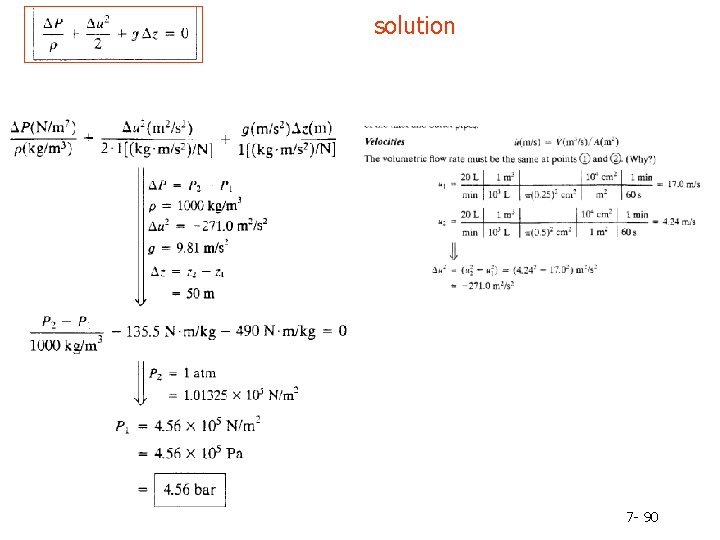
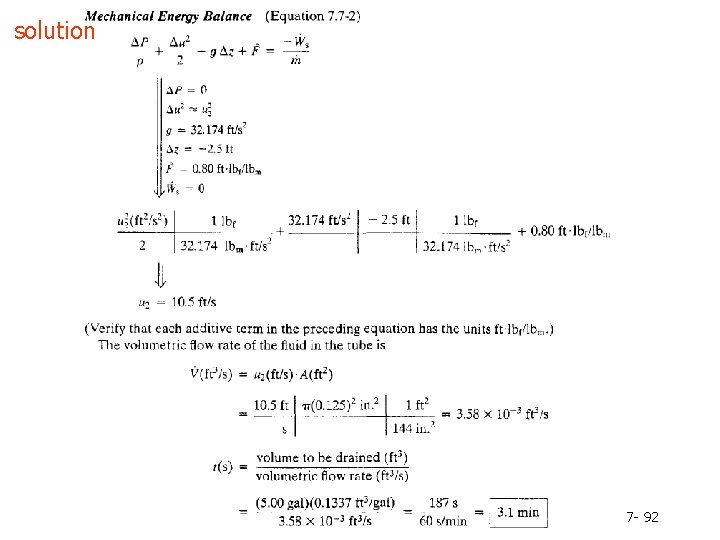
solution 7 - 90

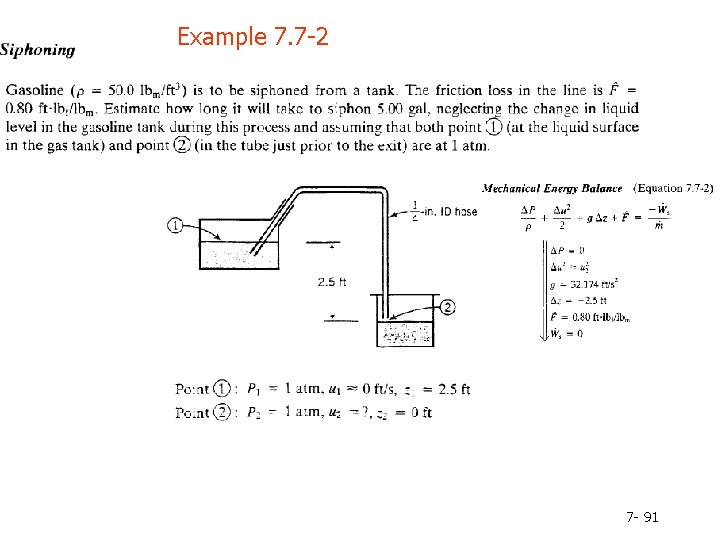
Example 7. 7 -2 7 - 91

solution 7 - 92

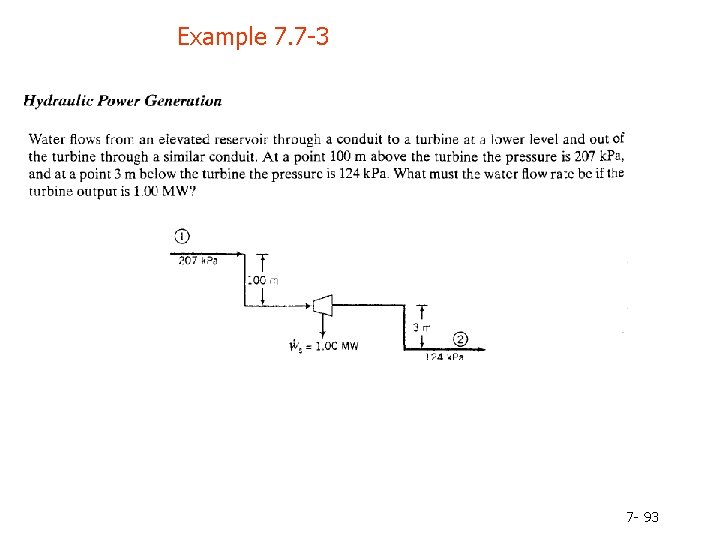
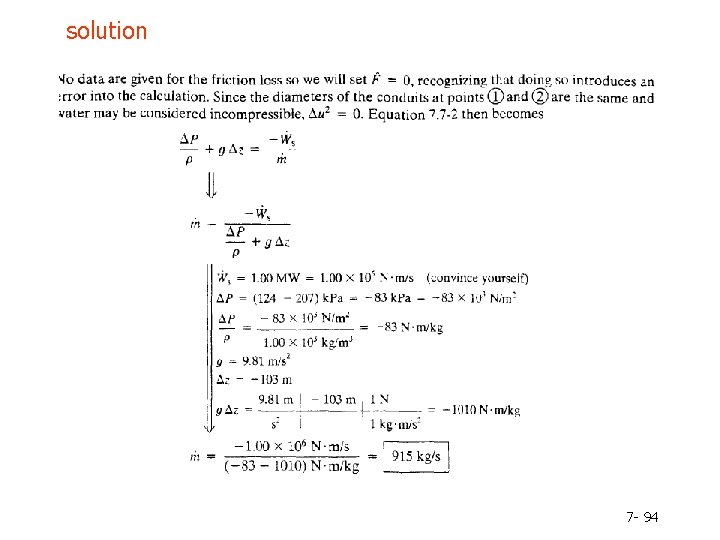
Example 7. 7 -3 7 - 93

solution 7 - 94