O18 Evaluation of the Prognostic and Predictive Significance



- Slides: 21

O-18 Evaluation of the Prognostic and Predictive Significance of Hepatocellular Carcinoma Circulating Tumor Cells Expressing Programmed Death-Ligand 1 (PD-L 1) Pin Jun Chen, Paul Winograd, Shuang Hou, Colin Court, Saeed Sadeghi, Richard Finn, Yazhen Zhu, Fady Kaldas, Ronald Busuttil, James Tomlinson, Hsian-Rong Tseng, Vatche G. Agopian 13 th Annual Conference 13 th 20 ► 22 September 2019 Chicago, USA ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

Nothing to Disclose 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

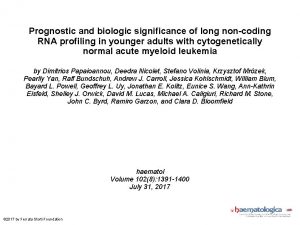
Hepatocellular Carcinoma: Epidemiology • HCC is the 6 th most common cancer and 3 rd leading cause of cancer death worldwide • HCC incidence and mortality continues to rise in the United States • Majority of patients present with surgically unresectable, incurable disease. Forner, A. , M. Reig, and J. Bruix, Lancet, 2018. Petrick JL, et al. , J Clin Oncol. 2016 May 20. 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA 3

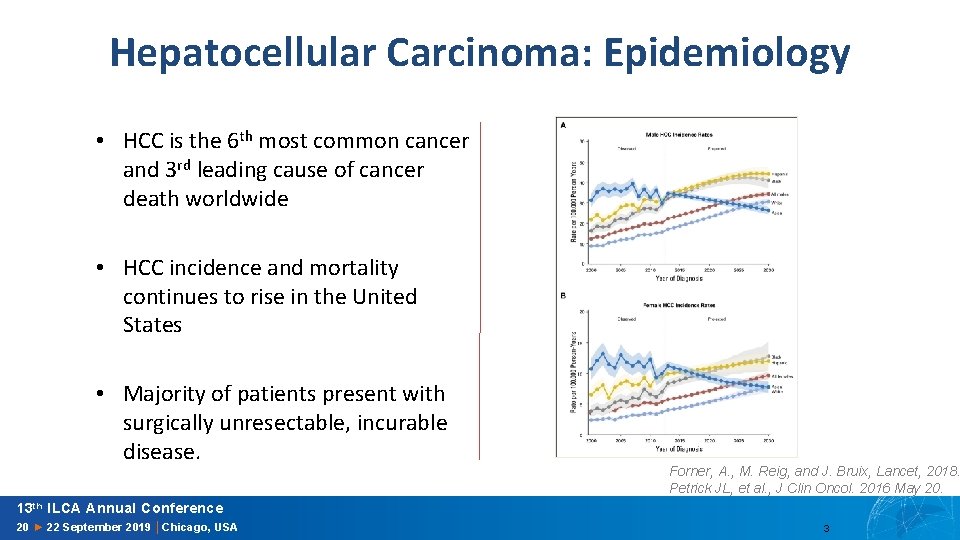
Hepatocellular Carcinoma: Systemic Treatment Sorafenib Regorafenib Lenvatinib Median OS 10. 7 ∆OS 2. 8 months Median OS 10. 6 ∆OS 2. 8 months Median OS 13. 6 ∆OS 1. 3 months Llovet, J. M. , et al. , N Engl J Med, 2008. Bruix, J. , et al. , Lancet, 2017. Kudo M et al. , Lancet 2018 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

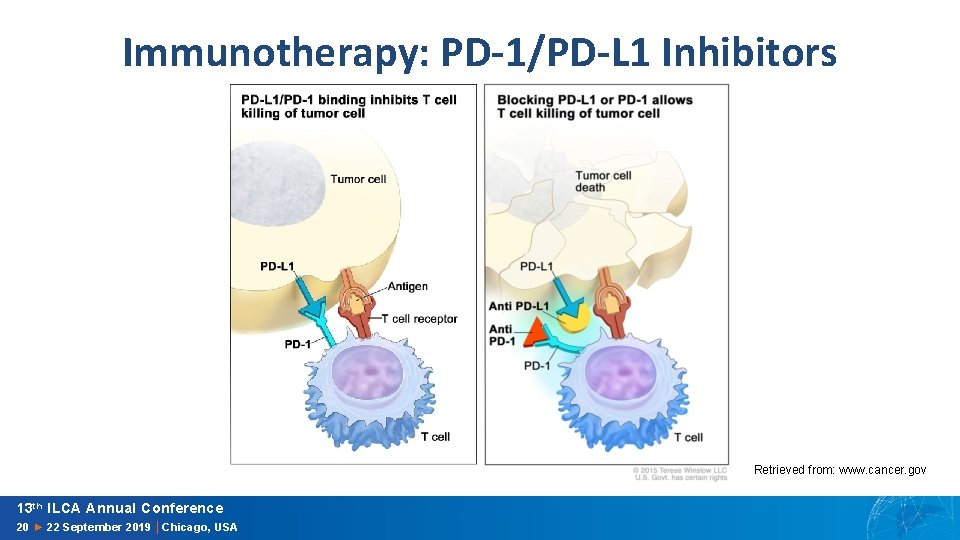
Immunotherapy: PD-1/PD-L 1 Inhibitors Retrieved from: www. cancer. gov 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

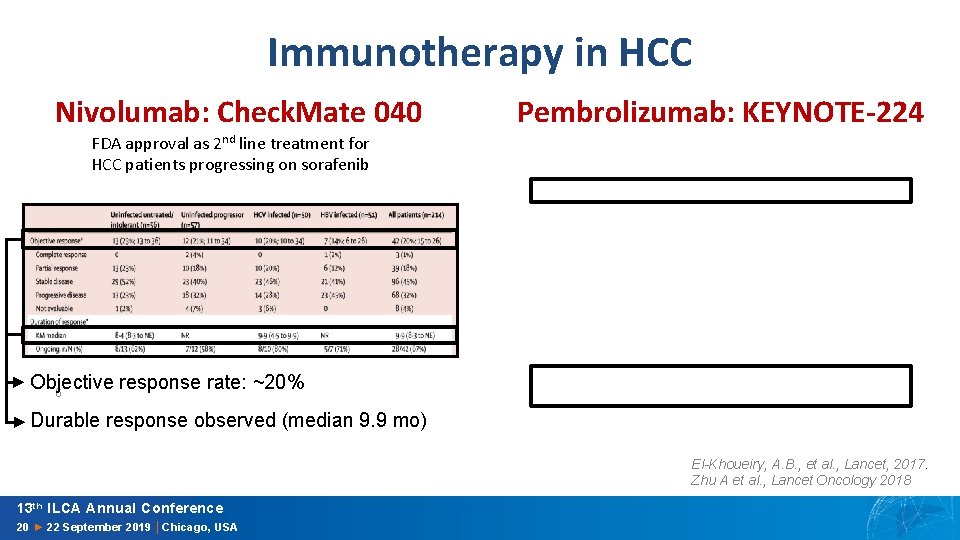
Immunotherapy in HCC Nivolumab: Check. Mate 040 Pembrolizumab: KEYNOTE-224 FDA approval as 2 nd line treatment for HCC patients progressing on sorafenib Objective response rate: ~20% 6 Durable response observed (median 9. 9 mo) El-Khoueiry, A. B. , et al. , Lancet, 2017. Zhu A et al. , Lancet Oncology 2018 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

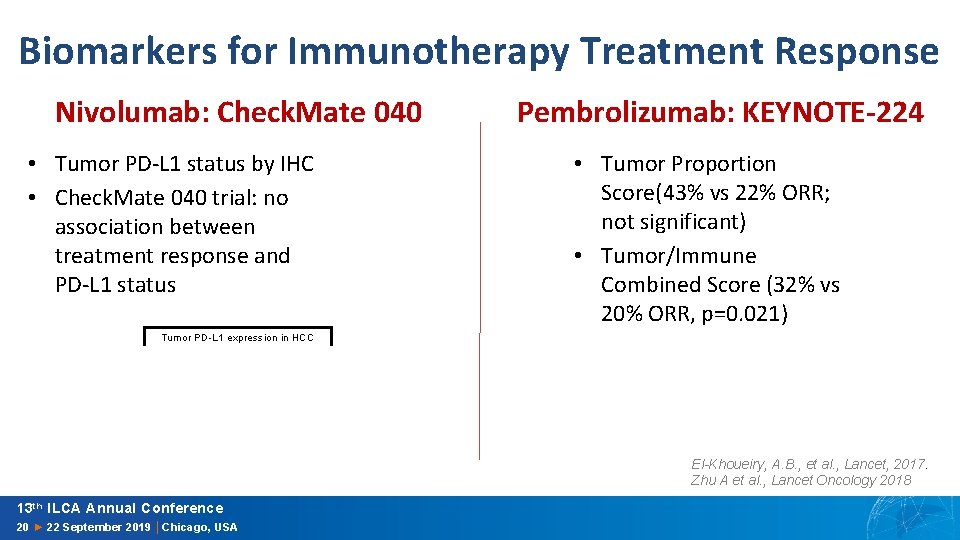
Biomarkers for Immunotherapy Treatment Response Nivolumab: Check. Mate 040 • Tumor PD-L 1 status by IHC • Check. Mate 040 trial: no association between treatment response and PD-L 1 status Pembrolizumab: KEYNOTE-224 • Tumor Proportion Score(43% vs 22% ORR; not significant) • Tumor/Immune Combined Score (32% vs 20% ORR, p=0. 021) Tumor PD-L 1 expression in HCC El-Khoueiry, A. B. , et al. , Lancet, 2017. Zhu A et al. , Lancet Oncology 2018 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

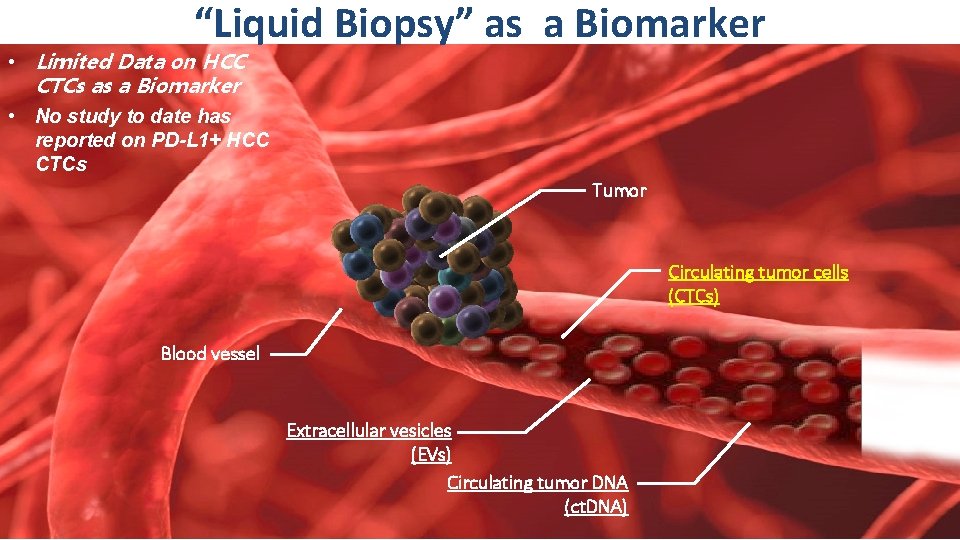
“Liquid Biopsy” as a Biomarker • Limited Data on HCC CTCs as a Biomarker • No study to date has reported on PD-L 1+ HCC CTCs Tumor Circulating tumor cells (CTCs) Blood vessel 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA Extracellular vesicles (EVs) Circulating tumor DNA (ct. DNA)

Specific Aims 1. Identify and enumerate HCC CTCs, and evaluate feasibility of phenotyping CTCs expressing PD-L 1 2. To evaluate the potential of PD-L 1+ CTCs to serve as a prognostic biomarker in discriminating early/advanced stage diseases and survival 3. Assess ability of PD-L 1+ CTCs as a predictive biomarker in a subset of patients undergoing anti-PD-1 therapy 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

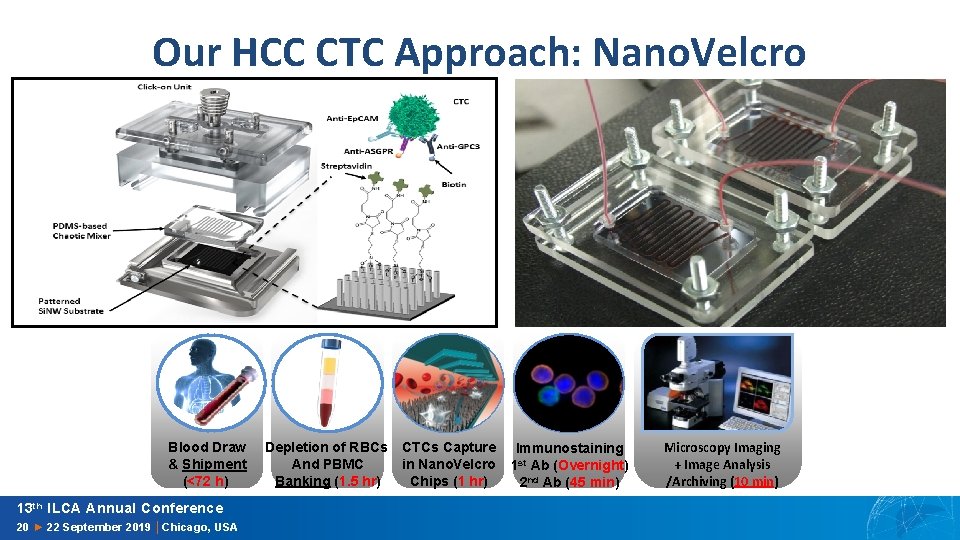
Our HCC CTC Approach: Nano. Velcro Blood Draw & Shipment (<72 h) 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA Depletion of RBCs And PBMC Banking (1. 5 hr) CTCs Capture in Nano. Velcro Chips (1 hr) Immunostaining 1 st Ab (Overnight) 2 nd Ab (45 min) Microscopy Imaging + Image Analysis /Archiving (10 min)

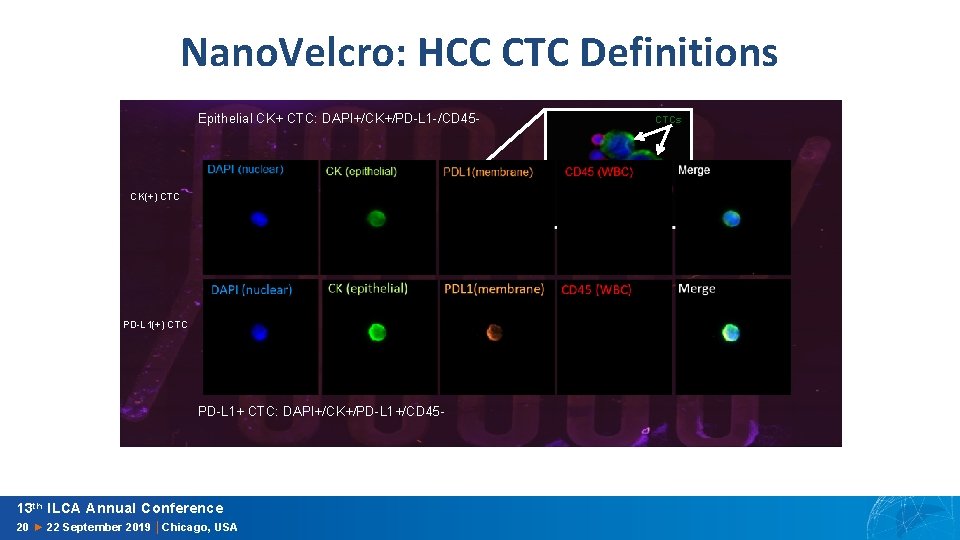
Nano. Velcro: HCC CTC Definitions Epithelial CK+ CTC: DAPI+/CK+/PD-L 1 -/CD 45 - CTCs CK(+) CTC WBCs PD-L 1(+) CTC PD-L 1+ CTC: DAPI+/CK+/PD-L 1+/CD 45 - 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

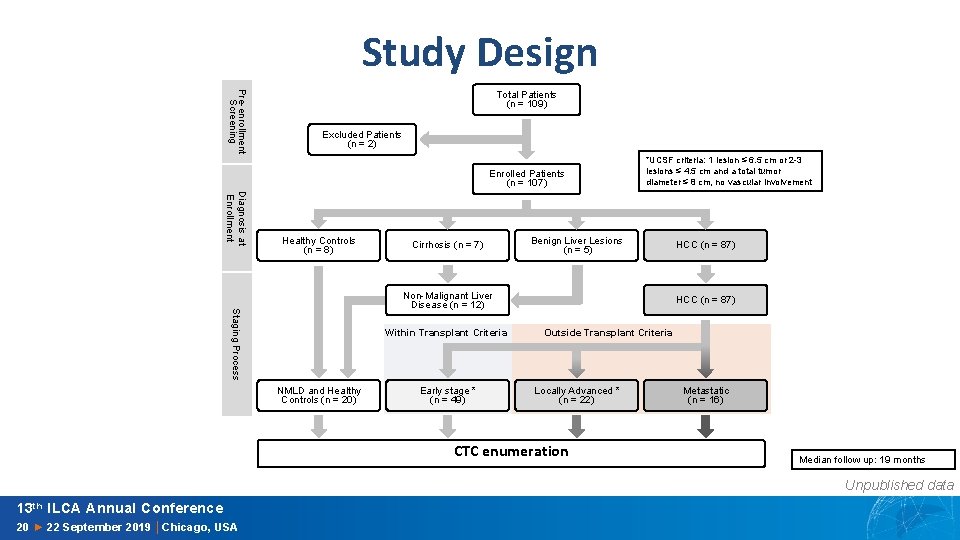
Study Design Pre-enrollment Screening Total Patients (n = 109) Excluded Patients (n = 2) Enrolled Patients (n = 107) Diagnosis at Enrollment Healthy Controls (n = 8) Cirrhosis (n = 7) *UCSF criteria: 1 lesion ≤ 6. 5 cm or 2 -3 lesions ≤ 4. 5 cm and a total tumor diameter ≤ 8 cm, no vascular involvement Benign Liver Lesions (n = 5) Staging Process Non-Malignant Liver Disease (n = 12) Within Transplant Criteria NMLD and Healthy Controls (n = 20) Early stage * (n = 49) HCC (n = 87) Outside Transplant Criteria Locally Advanced * (n = 22) CTC enumeration Metastatic (n = 16) Median follow up: 19 months Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

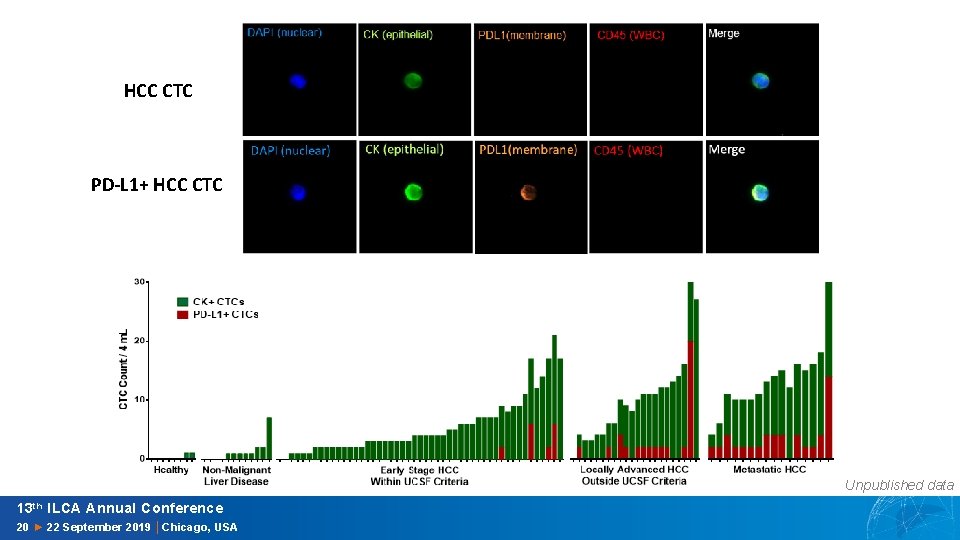
HCC CTC PD-L 1+ HCC CTC Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

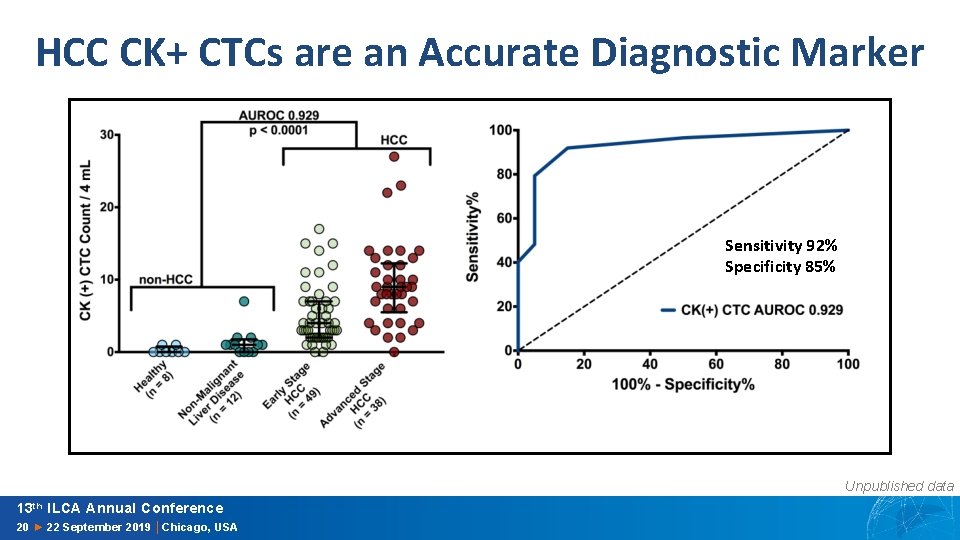
HCC CK+ CTCs are an Accurate Diagnostic Marker Sensitivity 92% Specificity 85% Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

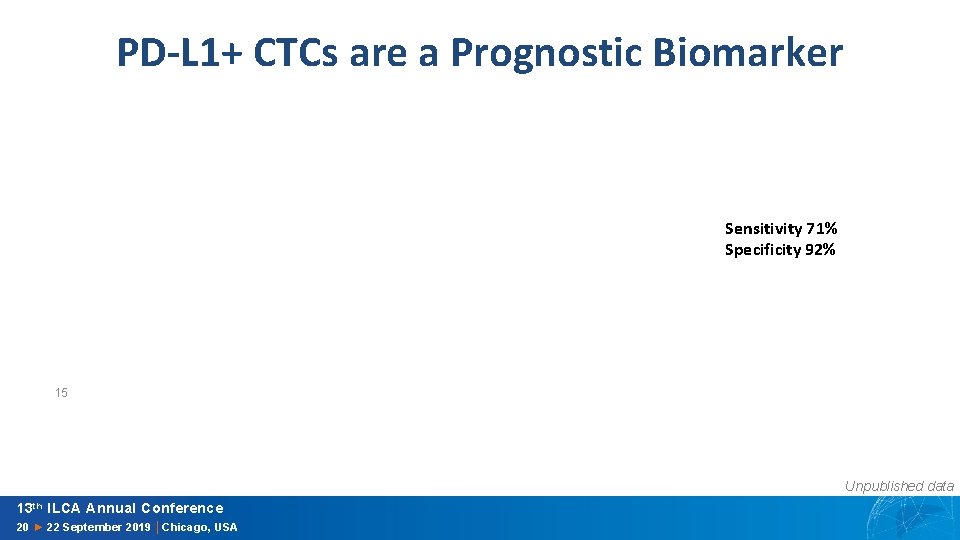
PD-L 1+ CTCs are a Prognostic Biomarker Sensitivity 71% Specificity 92% 15 Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

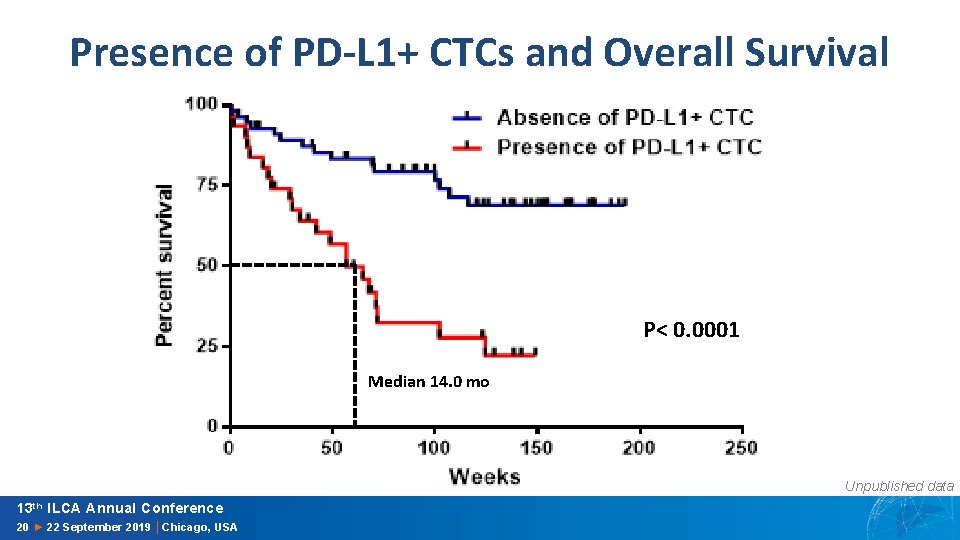
Presence of PD-L 1+ CTCs and Overall Survival P< 0. 0001 Median 14. 0 mo Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

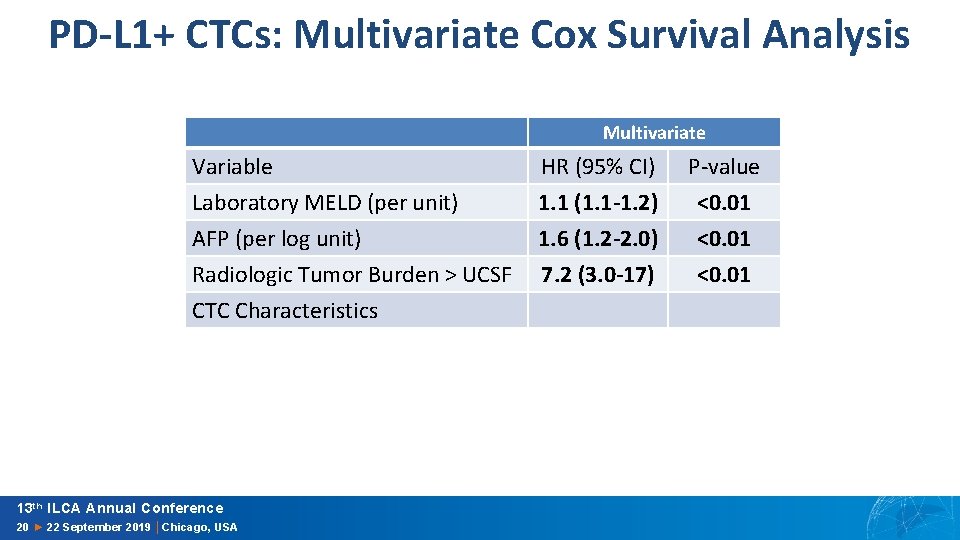
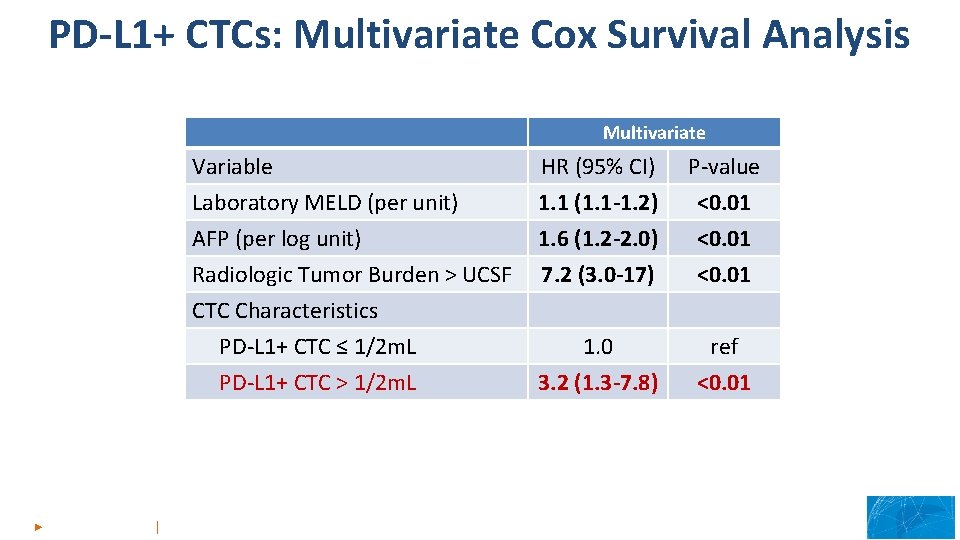
PD-L 1+ CTCs: Multivariate Cox Survival Analysis Multivariate Variable HR (95% CI) P-value Laboratory MELD (per unit) AFP (per log unit) Radiologic Tumor Burden > UCSF CTC Characteristics 1. 1 (1. 1 -1. 2) 1. 6 (1. 2 -2. 0) 7. 2 (3. 0 -17) <0. 01 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

PD-L 1+ CTCs: Multivariate Cox Survival Analysis Multivariate Variable HR (95% CI) P-value Laboratory MELD (per unit) AFP (per log unit) Radiologic Tumor Burden > UCSF CTC Characteristics 1. 1 (1. 1 -1. 2) 1. 6 (1. 2 -2. 0) 7. 2 (3. 0 -17) <0. 01 1. 0 3. 2 (1. 3 -7. 8) ref <0. 01 PD-L 1+ CTC ≤ 1/2 m. L PD-L 1+ CTC > 1/2 m. L 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

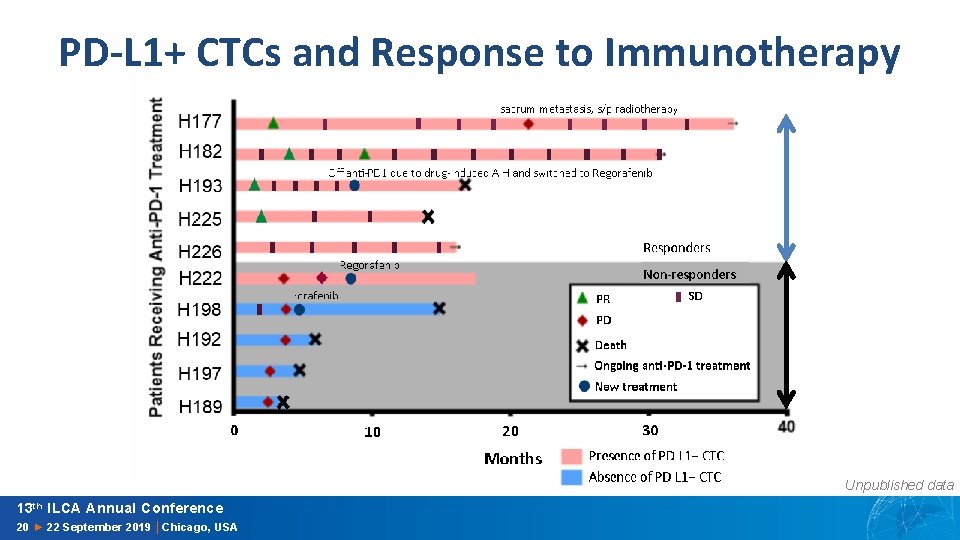
PD-L 1+ CTCs and Response to Immunotherapy Unpublished data 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

Summary • First study to characterize PD-L 1+ CTC phenotyping in HCC • PD-L 1+ CTCs are prognostic: – Discriminate early stage/curable and advanced stage/incurable HCC – Independently portend poor survival after controlling for MELD, AFP, and tumor stage • PD-L 1+ CTCs are potentially predictive: – Associated with treatment response to anti-PD 1 treatment 20 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA

Agopian LAB: Collaborators: Amy Chen Shuang Hou Colin Court Paul Winograd Daniela Markovic Thank You UCLA/Dumont LCC: UCLA Dept of Surgery: Ronald Busuttil, MD Ph. D Douglas Farmer, MD Hsian-Rong Tseng UCLA Division of Medical and Molecular Pharmacology Richard Finn UCLA Division of Hematology-Oncology Saeed Sadeghi UCLA Division of Hematology -Oncology Funding: American Surgical Association Foundation NIH/NCI R 21 CA 216807 (Agopian -PI) NIH/NCI R 21 CA 235340 (Agopian- co. PI) NIH/NCI R 01 CA 218486 (Agopian – site PI) NIH/NCI R 01 CA 204145 (Agopian – site PI) 13 th ILCA Annual Conference 20 ► 22 September 2019 │Chicago, USA Fady Kaldas, MD Mia Camcam, NP LCC Coordinator Joseph Di. Norcia, MD
 Surface analysis chart symbols
Surface analysis chart symbols Who prognostic scoring system gtn
Who prognostic scoring system gtn Who prognostic scoring system gtn
Who prognostic scoring system gtn Dyspermy
Dyspermy Palliative prognostic index
Palliative prognostic index Figo prognostic scoring system gtn
Figo prognostic scoring system gtn Van nuys prognostic index
Van nuys prognostic index Kcapanse
Kcapanse Preventive and predictive maintenance of hydro power plant
Preventive and predictive maintenance of hydro power plant Concurrent criterion validity
Concurrent criterion validity Health care risk adjustment and predictive modeling
Health care risk adjustment and predictive modeling Predictive analytics risk adjustment healthcare
Predictive analytics risk adjustment healthcare Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới