Nuclear medicine Essential idea Nuclear radiation whilst dangerous









- Slides: 23

Nuclear medicine Essential idea Nuclear radiation, whilst dangerous owing to its ability to damage cells and cause mutations, can also be used to both diagnose and cure diseases.

Radiotherapy • Use of ionizing radiation as emitted by radionuclides to treat diseases (radiotherapy) such as cancer by destroying the cancer cells. • Also used to diagnose a disease by providing detailed information in the form of images (nuclear imaging) about internal organs affected by the disease. • Radioisotopes have an unstable nucleus that decays spontaneously into a more stable form by emitting radiation either in the form of subatomic particles or energy.

Radiotherapy can be … • Internal: radionuclides are placed in the human body to target a particular cancerous tissue or group of receptors. Internal radionuclides are taken in orally as a solid or as a liquid, as an implant or injected in the case of some liquid radionuclides. • External: radiation source remains outside the human body and the beams of radiation (beta or gamma rays, photons or neutrons) target specific cancerous tissues in the body. A commonly used radionuclide for this approach is Co-60.

Effect of nuclear radiation on cells Removes electrons from atoms in biological molecules converting them into ions (ionizing radiation) that form reactive radicals (H and OH that interfere with physiological processes. This causes genetic damage which can lead to cell death, mutations and cancer: o Break structure of DNA. o Changes to the structure of the DNA within the genes of cells (genetic damage) (mutations). o A reduced ability of cells to repair DNA damage. o Limited growth and regeneration of the cells/tissue.

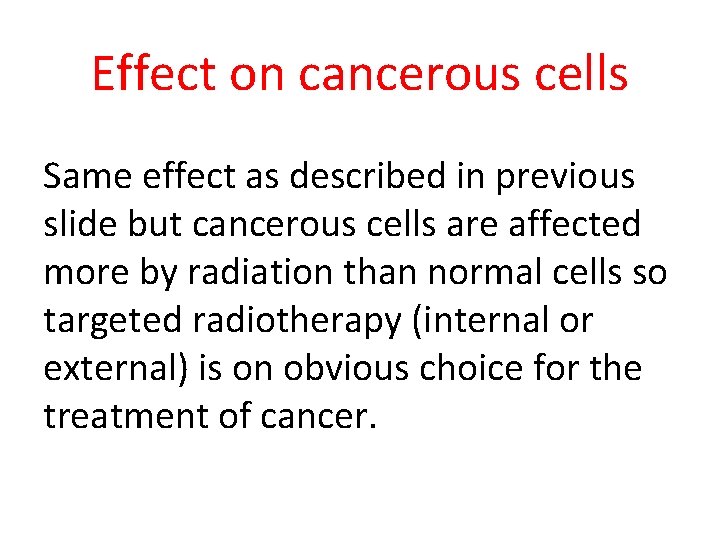
Effect on cancerous cells Same effect as described in previous slide but cancerous cells are affected more by radiation than normal cells so targeted radiotherapy (internal or external) is on obvious choice for the treatment of cancer.

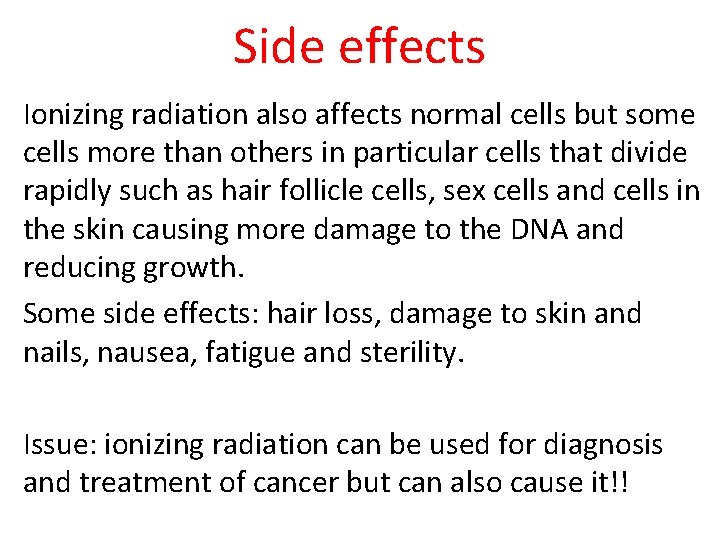
Side effects Ionizing radiation also affects normal cells but some cells more than others in particular cells that divide rapidly such as hair follicle cells, sex cells and cells in the skin causing more damage to the DNA and reducing growth. Some side effects: hair loss, damage to skin and nails, nausea, fatigue and sterility. Issue: ionizing radiation can be used for diagnosis and treatment of cancer but can also cause it!!

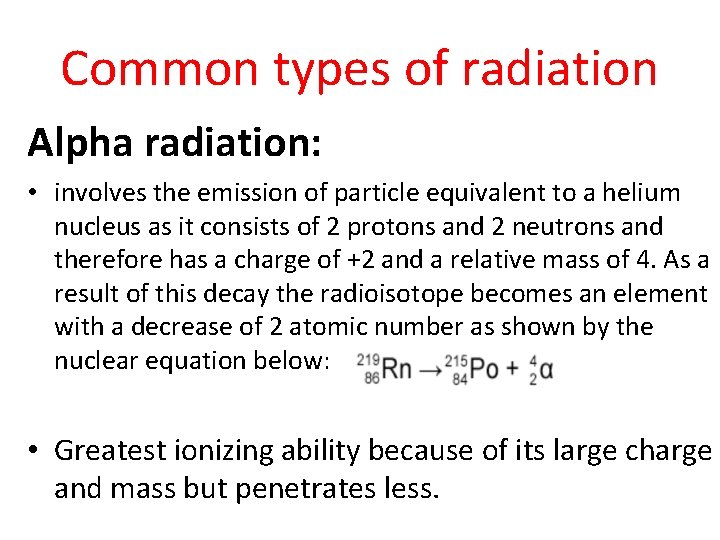
Common types of radiation Alpha radiation: • involves the emission of particle equivalent to a helium nucleus as it consists of 2 protons and 2 neutrons and therefore has a charge of +2 and a relative mass of 4. As a result of this decay the radioisotope becomes an element with a decrease of 2 atomic number as shown by the nuclear equation below: • Greatest ionizing ability because of its large charge and mass but penetrates less.

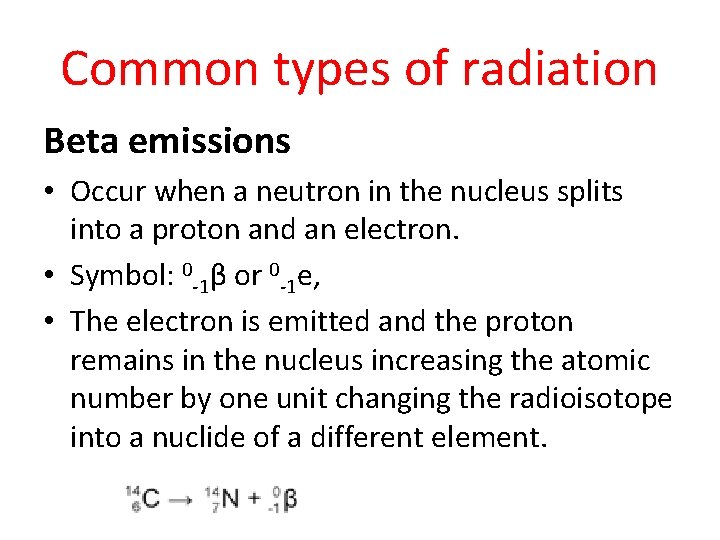
Common types of radiation Beta emissions • Occur when a neutron in the nucleus splits into a proton and an electron. • Symbol: 0 -1β or 0 -1 e, • The electron is emitted and the proton remains in the nucleus increasing the atomic number by one unit changing the radioisotope into a nuclide of a different element.

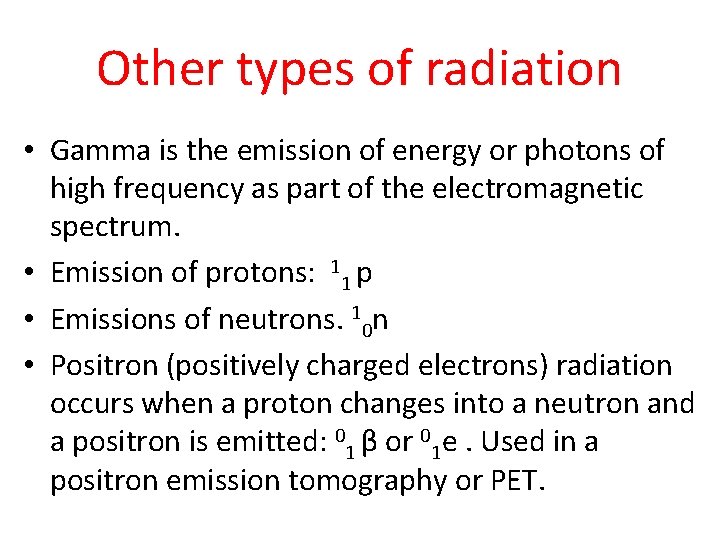
Other types of radiation • Gamma is the emission of energy or photons of high frequency as part of the electromagnetic spectrum. • Emission of protons: 11 p • Emissions of neutrons. 10 n • Positron (positively charged electrons) radiation occurs when a proton changes into a neutron and a positron is emitted: 01 β or 01 e. Used in a positron emission tomography or PET.

Exercises on nuclear equations Write nuclear equations for the following: • Alpha decay of Pb-212 • Beta decay of Y-90 • Beta decay of Lu-177 • Alpha decay of Ac-255 • Beta decay of Co-60 • Beta decay of Ca-40

Targeted alpha therapy (TAT) • Used for treating leukemia and other dispersed cancers; these are cancers in which the cells have spread throughout the body from the original tumour. As alpha particles have the greatest charge and therefore the greatest ionizing ability, they are the most destructive type of emission. Alpha particles can only penetrate tissue, and therefore cause cellular damage, over a very short range of 0. 05 mm 0. 1 mm. This also means that not too many healthy cells should be effected. • The radionuclides emitting the alpha radiation are directed to antibodies and bind themselves onto them. The antibody then carries the alpha-emitter to the cancer tissue where the alpha radiation will destroy the cells without too much damage to the surrounding healthy cells.

Boron Neutron Capture Therapy (BNCT) This therapy uses a beam of neutrons to produce alpha particles only at the site of the cancer. The target of the external neutron radiation are B-10 atoms that have been taken to the site of the cancer. There the B-10 atoms capture (absorb) the neutrons from the beam and then change into B-11 nucleii; these then immediately decay emitting alpha particles that destroy the surrounding cancerous cells. The B-10 nucleii are administered using intravenous injections in the form of a compound such as boronophenylalanine. This compound tends to accumulate in brain tumours. When the compound has been absorbed by the tumour cells the site is radiated by a neutron beam.

BNCT Write a nuclear equation of: o The neutron capture of B-10

Radiodiagnostics • Use of a radioactive tracer that is attached to a biologically active molecule to form a radiopharmaceutical • Radiopharmaceutical is ingested or injected • Traced used detection equipment that uses for instance gamma rays producing an image on a scan. • Commonly used tracers: o Tc-99 m (most common) o I-131. Different traces accumulate in different parts of the body e. g. I-131 in the thyroid gland which is why it is used in the diagnosis and treatment (higher dose than for diagnostic purposes) of thyroid cancer.

Tc-99 m diagnostic nuclide: Why? • Emits gamma radiation: o Easily traced as it emits gamma radiation; detected using Xrays. o As source of the emission is inside body can only be detected if the radiation can escape the body which gamma rays can. o Gamma = low energy radiation so less hazardous to the patient: only affected by a low dose. • Versatile and easily administered: o Can be used to diagnose and treat cancer in different organs and tissues as it can bind onto a number of different biological carrier. Each type of tissue has its own biologically active molecule that accumulates there. o Can easily be administered to specific areas in the body. • Patient is only exposed to a minimum amount of radiation: half life is 6 hours and most of Tc-99 m will have decayed after 2 days. • Short biological half life; leaves body after 1 day. • Beta radiation: low energy electrons.

Lu-177 • Emits strong beta radiation • Also emits gamma radiation that can be detected outside the body and can therefore be used for imaging.

Y-90 • Emits strong beta radiation • Also emits gamma radiation that can be detected outside the body and can therefore be used for imaging. • Short half life of 64 hours

Activity or rate of decay Rate of decay is the number of nuclides that decay or emit radiation per second. The unit is Bequerel or Bq.

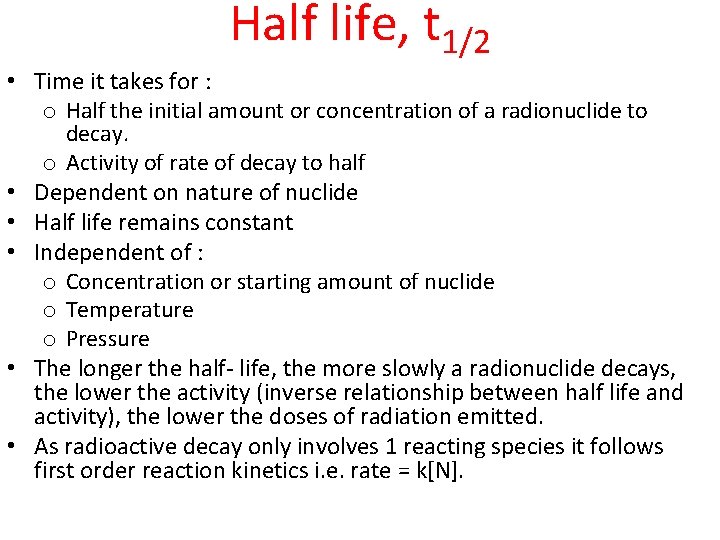
Half life, t 1/2 • Time it takes for : o Half the initial amount or concentration of a radionuclide to • • • decay. o Activity of rate of decay to half Dependent on nature of nuclide Half life remains constant Independent of : o Concentration or starting amount of nuclide o Temperature o Pressure The longer the half- life, the more slowly a radionuclide decays, the lower the activity (inverse relationship between half life and activity), the lower the doses of radiation emitted. As radioactive decay only involves 1 reacting species it follows first order reaction kinetics i. e. rate = k[N].

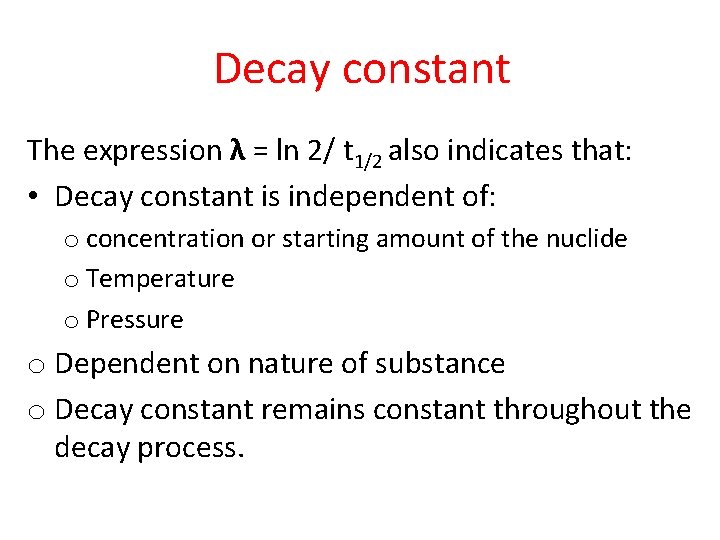
Decay constant The expression λ = ln 2/ t 1/2 also indicates that: • Decay constant is independent of: o concentration or starting amount of the nuclide o Temperature o Pressure o Dependent on nature of substance o Decay constant remains constant throughout the decay process.

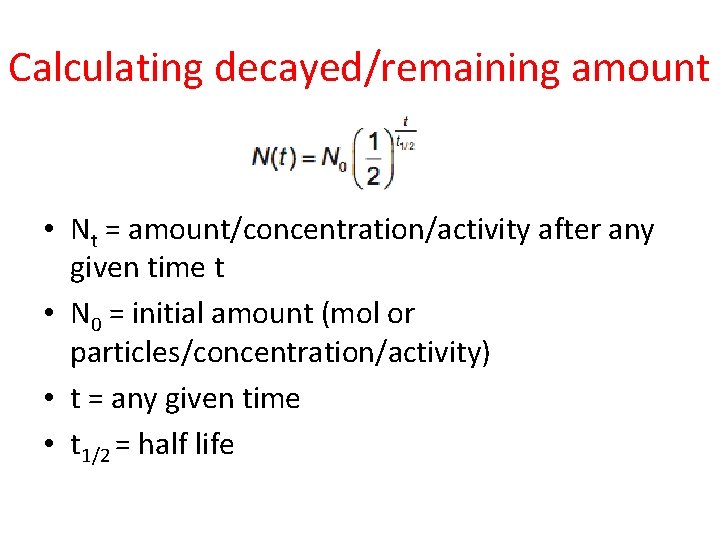
Calculating decayed/remaining amount • Nt = amount/concentration/activity after any given time t • N 0 = initial amount (mol or particles/concentration/activity) • t = any given time • t 1/2 = half life

Medical considerations Long half life means low value for decay constant and low value for activity. Nuclides used in diagnosis or therapy should have shorter half lives so they do not remain radioactive for too long but not too short either as otherwise patient will receive high dose of radiation. Long half life is also not ideal as often nuclides remain in the body for a while and will make patient be exposed to radiation for a longer period of time.

MRI • Magnetic resonance imaging. • MRI uses the same principles as NMR as the scans use very powerful magnets to detect not just H-1 but also C-13, Na-23, He-3 and P-31 nuclei and also use low frequency radio waves that are not ionizing therefore no known impact on human tissue – non-invasive. • Protons in different tissues absorb radiowaves of different frequencies e. g. cancerous tissue; computers identify the type of tissue a proton is in. • The radiowaves absorbed by the nuclei are detected and used by computers to identify the type of tissue the proton is in and produce 2 D or 3 D images of internal organs or body parts.
 Gap year poem
Gap year poem Nuclear radiation
Nuclear radiation Nuclear fusion radiation
Nuclear fusion radiation Types of radiation
Types of radiation What is nuclear radiation
What is nuclear radiation Plamatic acid
Plamatic acid Diaphragm
Diaphragm Nuclear medicine information system
Nuclear medicine information system Nuclear medicine lectures
Nuclear medicine lectures Case presentation introduction
Case presentation introduction Venus in medical terms
Venus in medical terms Measles artifact nuclear medicine
Measles artifact nuclear medicine Spatial resolution
Spatial resolution Mt sinai nuclear medicine
Mt sinai nuclear medicine Filtered back projection
Filtered back projection Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Essential idea
Essential idea What is the implied main idea of the passage?
What is the implied main idea of the passage? Contoh topik dan controlling idea
Contoh topik dan controlling idea Wacana penulisan gwp 1092
Wacana penulisan gwp 1092 Idea subject
Idea subject Theme vs. central idea
Theme vs. central idea What are irrelevant sentences
What are irrelevant sentences