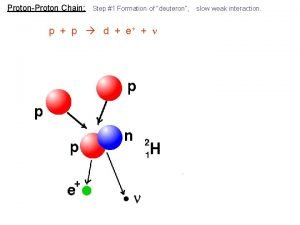
Nuclear Energy Nuclear Fission the splitting of two
















- Slides: 31


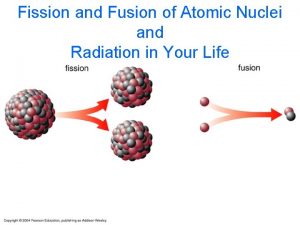
Nuclear Energy • Nuclear Fission - the splitting of two atoms • Nuclear fusion – the combining of 2 atoms • Both processes release energy

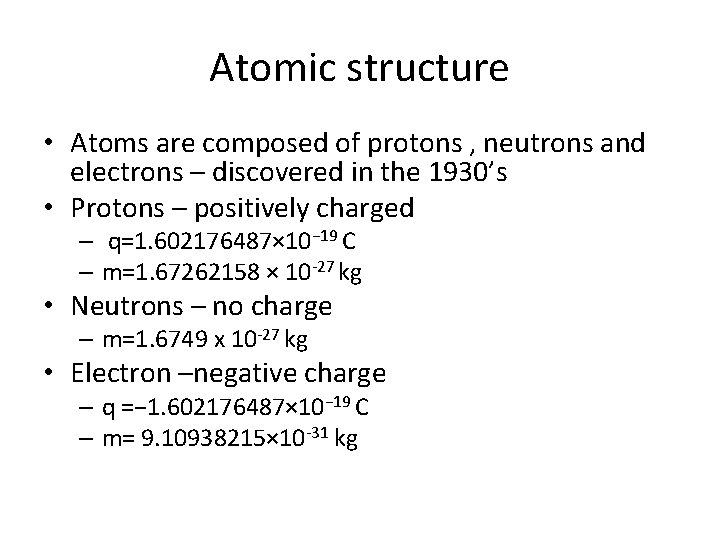
Atomic structure • Atoms are composed of protons , neutrons and electrons – discovered in the 1930’s • Protons – positively charged – q=1. 602176487× 10− 19 C – m=1. 67262158 × 10 -27 kg • Neutrons – no charge – m=1. 6749 x 10 -27 kg • Electron –negative charge – q =− 1. 602176487× 10− 19 C – m= 9. 10938215× 10 -31 kg

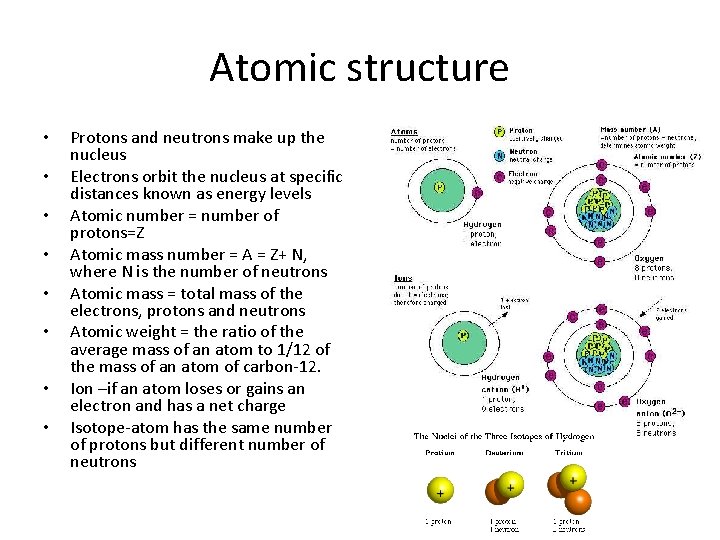
Atomic structure • • Protons and neutrons make up the nucleus Electrons orbit the nucleus at specific distances known as energy levels Atomic number = number of protons=Z Atomic mass number = A = Z+ N, where N is the number of neutrons Atomic mass = total mass of the electrons, protons and neutrons Atomic weight = the ratio of the average mass of an atom to 1/12 of the mass of an atom of carbon-12. Ion –if an atom loses or gains an electron and has a net charge Isotope-atom has the same number of protons but different number of neutrons

Atomic Structure • Atoms are held together by forces • There are four forces in nature – Gravity -force between masses – Electrostatic forces-like charges repel, unlike charges attract – Strong nuclear force - causes an attraction between protons and neutrons – Weak nuclear force – causes protons to transform into neutrons and neutrons in to protons

Atomic Structure • In atoms, the electrostatic force is holding the electrons to the nucleus, since the electrons are negatively charged and the protons are positively charged. • The protons in the nucleus are being pushed apart by the electrostatic force since they have the same charge, but the strong nuclear force overcomes this repulsion on the atomic size scales and holds the protons together.

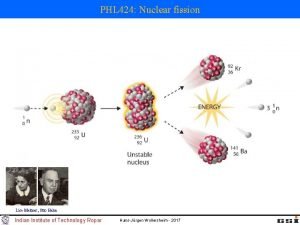
Fission • In the late 1930 s, it was discovered that if a uranium nucleus was bombarded by neutrons, it absorbed the neutron and became an unstable isotope of uranium, which then spilt into 2 separate atoms (Krypton and Barium) and emitted more neutrons and gamma rays

Is mass conserved? • Now the mass of the fission products plus the excess neutron should equal the mass of the initial incident neutron and the uranium. But it doesn’t. • Where did the mass go? • Well, remember E = mc 2 - mass cannot be created or destroyed, only converted to and from energy, so the missing mass must be converted into energy.

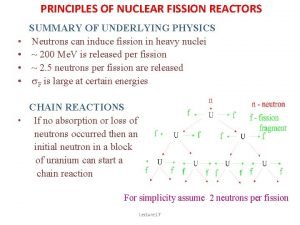
How much energy • 200 Me. V is released per fission event • The fission of 1 g of uranium or plutonium per day liberates about 1 MW. • This is the energy equivalent of 3 tons of coal or about 600 gallons of fuel oil per day • No CO 2 emissions! • Vastly superior in terms of energy per amount of fuel

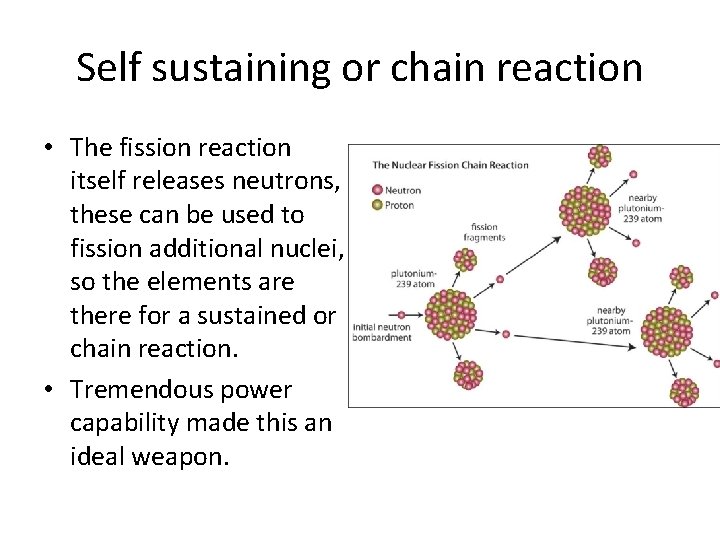
Self sustaining or chain reaction • The fission reaction itself releases neutrons, these can be used to fission additional nuclei, so the elements are there for a sustained or chain reaction. • Tremendous power capability made this an ideal weapon.

Fission Bombs • Created in response to a fear that Nazi Germany would develop one first, which would tip the balance of power and possibly the outcome of WWII in their favor. • Manhattan Project – Secret US project to develop a nuclear weapon • Developed 3 nuclear devices, one with 235 U and two with 239 PU. • One was tested in New Mexico in 1945, the other two were dropped on Hiroshima and Nagasaki Japan in August 1945, ending WWII in the Pacific.

Critical Mass • In order to sustain a chain reaction, one needs a specific amount of fissionable material, called the critical mass • Critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. • Creating the critical mass was one of the challenges that faced the Manhattan project

The devices • Fat Man and Little Boy • Little Boy – device dropped on Hiroshima • Gun-type device – One mass of U-235, the "bullet, " is fired down a gun barrel into another mass of U-235, rapidly creating the critical mass of U-235, resulting in an explosion.

The devices • • Fat Man Tested in New Mexico and dropped on Nagasaki Used Plutonium rather than Uranium Implosion style device – The required implosion was achieved by using shaped charges with many explosive lenses to produce the perfectly spherical explosive wave which compressed the plutonium sphere.

Effects of a Fission explosion • • • Blast Damage Thermal radiation Electromagnetic Pulse Ionizing radiation earthquake

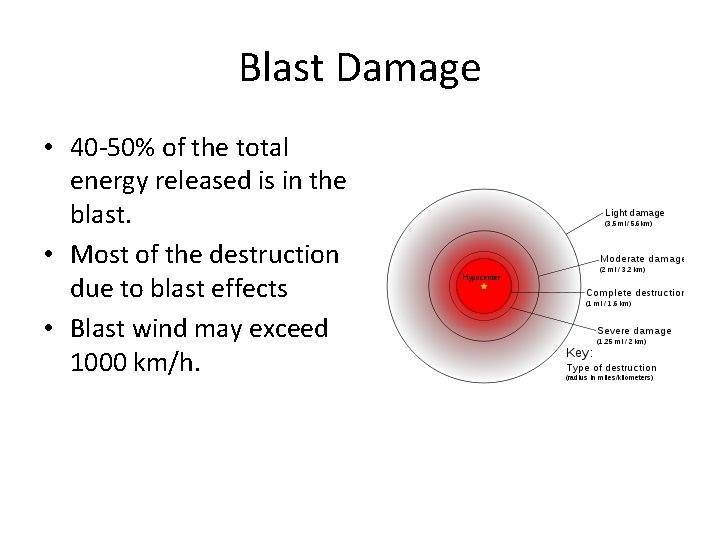
Blast Damage • 40 -50% of the total energy released is in the blast. • Most of the destruction due to blast effects • Blast wind may exceed 1000 km/h.

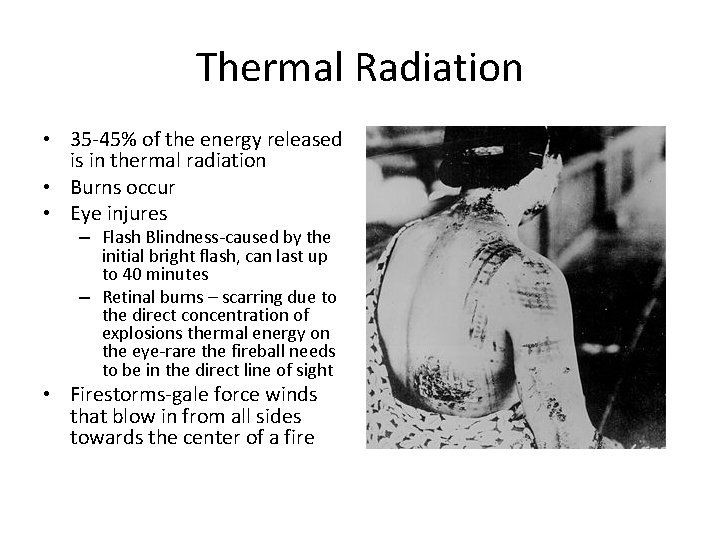
Thermal Radiation • 35 -45% of the energy released is in thermal radiation • Burns occur • Eye injures – Flash Blindness-caused by the initial bright flash, can last up to 40 minutes – Retinal burns – scarring due to the direct concentration of explosions thermal energy on the eye-rare the fireball needs to be in the direct line of sight • Firestorms-gale force winds that blow in from all sides towards the center of a fire

Electromagnetic pulse • The nuclear explosion produced high energy electromagnetic radiation-Gamma rays. • The Gamma rays interact with (scatter) electrons and produce higher energy electrons. • Long metal objects (cables, etc) act as antenna and generate high voltages and currents, which can damage or destroy electrical equipment. • No known biological effects, though useful against Sentinels (The Matrix).

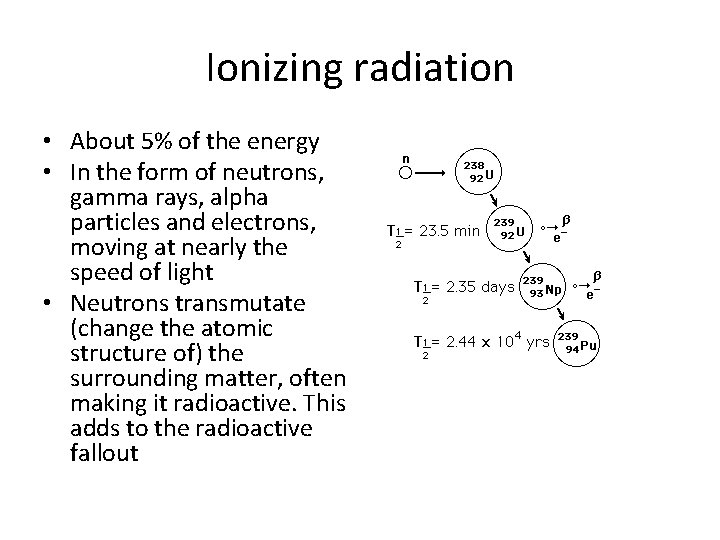
Ionizing radiation • About 5% of the energy • In the form of neutrons, gamma rays, alpha particles and electrons, moving at nearly the speed of light • Neutrons transmutate (change the atomic structure of) the surrounding matter, often making it radioactive. This adds to the radioactive fallout

What does this have to do with Nuclear Energy • It sets the historical context and shows the power released • To point out that this is NOT what will happen if there is an accident in a nuclear power facility-they do not “blow up” like explosive nuclear devices. More on that later

Nuclear reactor • In a nuclear power plant, the energy to heat the water to create steam to drive the turbine is provided by the fission of uranium, rather than the burning of coal. • Fuel is 3% 235 U and 97% 238 U. 235 U is an isotope of 238 U. The chain reaction will only occur in the 235 U, but naturally occurring uranium has both present in it. • The neutrons coming from a fission reaction have an energy of 2 Mev. They are too energetic to sustain a nuclear reaction in 235 U. • Need to slow them down to energies on the order of 10 -2 so they can sustain fission in the 235 U

Slowing the neutrons down • A moderator is used to slow down the neutrons and cause them to lose energy • The moderator could be water or graphite • The lower energy neutrons are called thermal neutrons • Some of the neutrons will be absorbed by 235 U instead of causing a fission reaction or by 238 U and resulting in the emission of a gamma ray in both cases. • Absorption of a neutron by 238 U can result in the creation of 239 Pu which is also fissionable

Creating Plutonium • So: 238 U captures a neutron creating 239 U • 239 U undergoes a beta decay in with a half life of 24 minutes and becomes 239 Np (Neptunium) • 239 Np then beta decays with a half life of 2. 3 days into 239 Pu. • 239 Pu has a half life of 24, 000 years • 239 Pu can also undergo fission by the slow neutrons in the core, with an even higher probability • So as it builds up in the core, is contributes to the fission reaction

Breeder reactor • A reactor designed to produce more fuel (usually 239 Pu ) than it consumes. • 239 Pu does not occur naturally, and it is more fissile than 235 U. • Leads to the possibility of reactors that can create their own fuel, and only need limited mounts of naturally occurring uranium to operate. • Also leads to the danger of countries creating additional nuclear fuels for weapons development – Caution-reactor must be designed to produce weapons grade plutonium, jut because someone has a nuclear reactor does not mean they create weapons grade plutonium

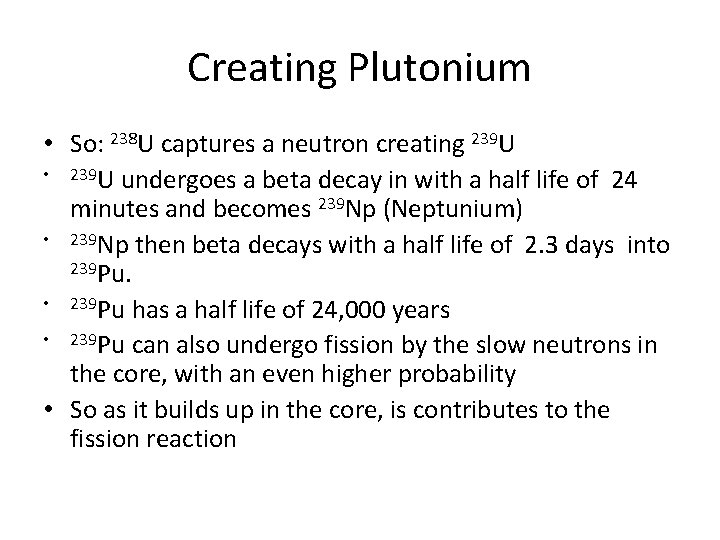
Reactor design • • PWR – pressurized water reactor Core – where the action is. Fuel assembly is kept in here (fuel is usually in the form of fuel rods) Fuel rods are surrounded by the water which acts as the moderator. This water is kept under high pressure so it never boils-it heats a seconds water source which turns into steam Control rods are slid in and out from the top to control the fission rate-in an emergency they can be dropped completely into the reactor core, quenching the fission Once the steam is generated, this works just like a fossil fuel power plant Can run without refueling for up to 15 years if the initial fuel is highly enriched Used in submarines and commercial power systems

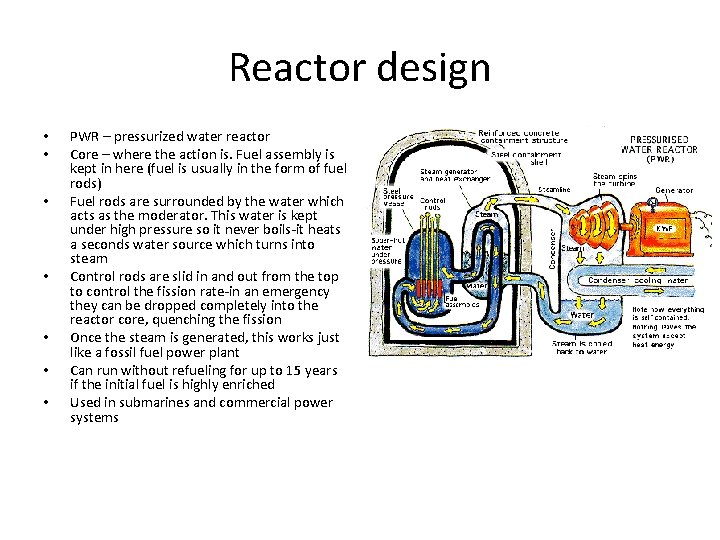
Reactor design • • • BWR –Boiling water reactor Core – where the action is. Fuel assembly is kept in here (fuel is usually in the form of fuel rods) Fuel rods are surrounded by the water which acts as the moderator and the source of steam Control rods are slid in and out from the bottom to control the fission rate -in an emergency they can be dropped completely into the reactor core, quenching the fission. Also, boron can be added to the water which also efficiently absorbs neutron Once the steam is generated, this works just like a fossil fuel power plant

Fuel Cycle • Fuel rods typically stay in a reactor about 3 years • When they are removed, they are thermally and radioactively hot • To thermally cool them they are put in a cooling pond. • Initial idea was that they would stay in the cooling pond for 150 days, then be transferred to a facility which would reprocess the uranium and plutonium for future use.

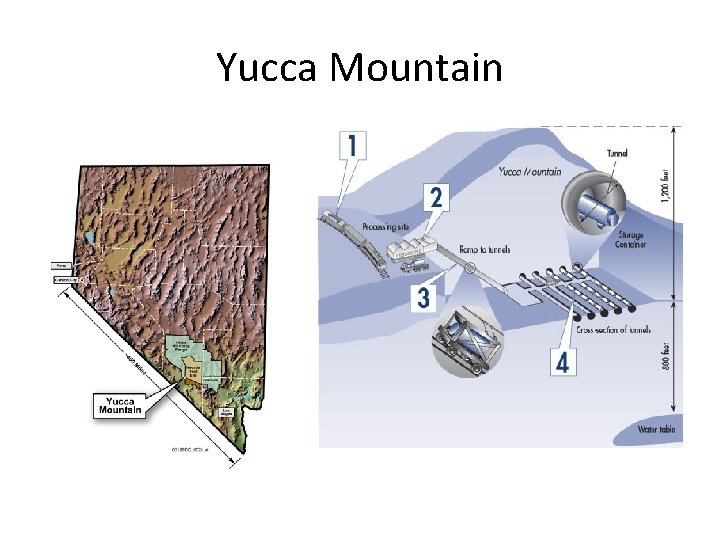
Nuclear waste disposal • This idea ran into problems. • Fear that the plutonium would be easily available for weapons use halted reprocessing efforts in 1977 • Note that it is very difficult to extract weapons grade plutonium from spent fuel rods • Plan is now to bury the waste deep underground, in a place called Yucca Mountain, Nevada

Nuclear waste The spent fuel rods are radioactive Radioactivity is measured in curies A curie is 3. 7 x 1010 decays per second A 1000 MW reactor would have 70 megacuries(MCI) of radioactive waste once it was shut down • After 10 years, this has decayed to 14 MCi • After 100 years, it is 1. 4 MCi • After 100, 000 years it is 2000 Ci • •

Yucca Mountain

 Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Nuclear fission and fusion
Nuclear fission and fusion Nuclear fission lise meitner
Nuclear fission lise meitner Who discovered uranium
Who discovered uranium Nuclear fission equation
Nuclear fission equation Fission and fusion similarities
Fission and fusion similarities Is the sun fusion or fission
Is the sun fusion or fission Fusion fission
Fusion fission Nuclear fission explanation
Nuclear fission explanation Fission vs fusion
Fission vs fusion Nuclear fission radiation
Nuclear fission radiation Pluotnium
Pluotnium Fission reaction
Fission reaction Fission equation
Fission equation Nuclear fission equation
Nuclear fission equation Venn diagram nuclear fission and fusion
Venn diagram nuclear fission and fusion Nuclear energy
Nuclear energy Are all things made of atoms
Are all things made of atoms Energy splitting
Energy splitting Fission vs fusion
Fission vs fusion Equivalenc
Equivalenc Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Bpd splitting meaning
Bpd splitting meaning Trauma bonding definition
Trauma bonding definition Medan kristal
Medan kristal Splitting of s2
Splitting of s2 Fixed split s2 causes
Fixed split s2 causes Attachment style chart
Attachment style chart Examples of passive-aggressive
Examples of passive-aggressive