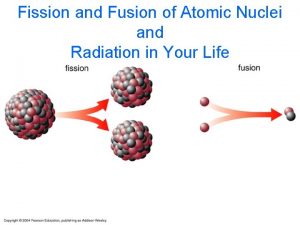
Splitting The Atom Nuclear Fission Fission Large mass


















- Slides: 38

Splitting The Atom Nuclear Fission

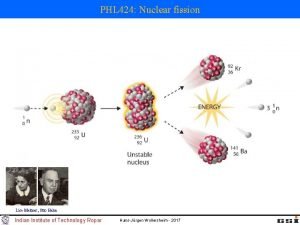
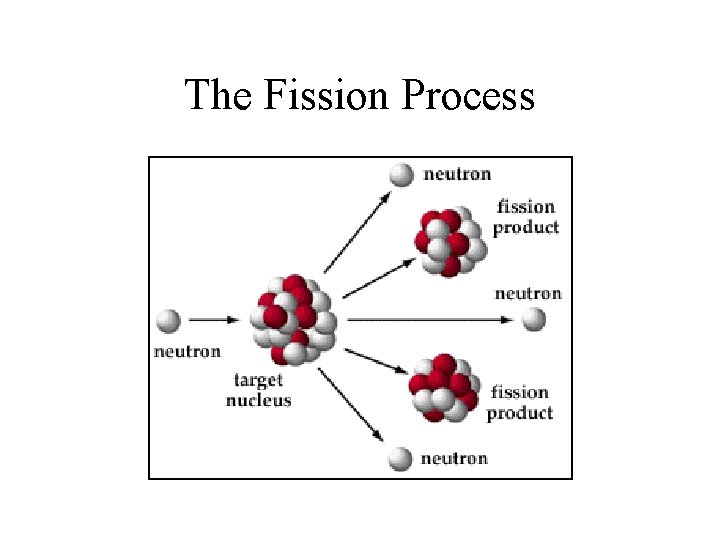
Fission • Large mass nuclei split into two or more smaller mass nuclei – Preferably mass numbers closer to 56 • Neutrons also emitted • Fission can occur when – unstable large mass atom captures a neutron – can happen spontaneously

Fission is Exothermic • Masses resulting nuclei < original mass Ø~0. 1% less • “Missing mass” converted into energy ØE = mc 2 • Large quantities of energy are released because the products are higher up the Binding Energy Curve

The Fission Process

Energy Released By A Fission • U + n --> fission + 2 or 3 n + 200 Me. V 1 Me. V (million electron volts) = 1. 609 x 10 -13 j • This corresponds to 3. 2 x 10 -11 j • Production of one molecule of CO 2 in fossil fuel combustion only generates 4 ev or 6. 5 x 10 -19 j of energy • This is 50, 000 times more energy

Fissile Nuclei • Not all nuclei are capable of absorbing a neutron and then undergoing a fission reaction • U-235 and Pu-239 do undergo induced fission reactions • U-238 does not directly undergo an induced fission reaction

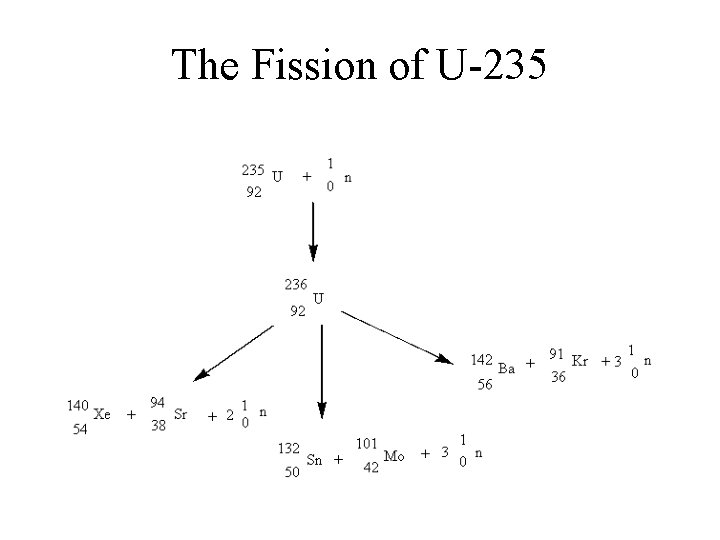
The Fission of U-235

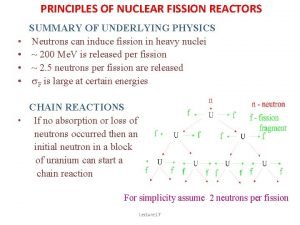
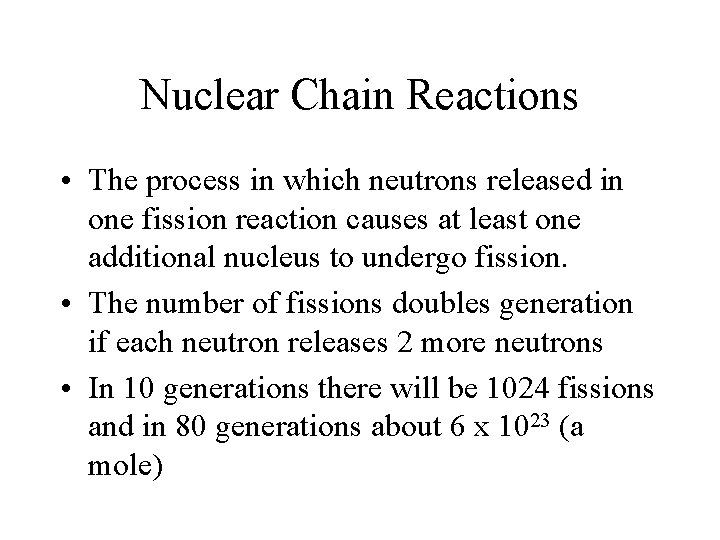
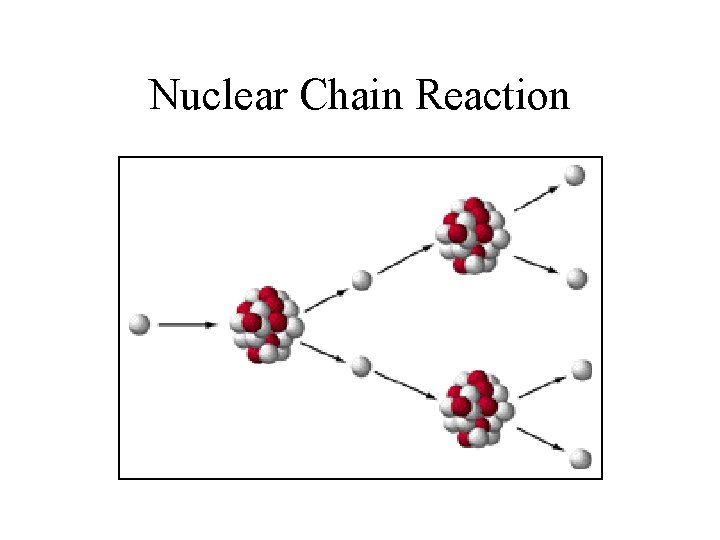
Nuclear Chain Reactions • The process in which neutrons released in one fission reaction causes at least one additional nucleus to undergo fission. • The number of fissions doubles generation if each neutron releases 2 more neutrons • In 10 generations there will be 1024 fissions and in 80 generations about 6 x 1023 (a mole)

Nuclear Chain Reaction

Critical Mass • If the amount of fissile material is small, many of the neutrons will not strike another nucleus and the chain reaction will stop • The critical mass is the amount of fissile material necessary for a chain reaction to become self-sustaining.

Nuclear Chain Reactions • An uncontrolled chain reaction is used in nuclear weapons • A controlled chain reaction can be used for nuclear power generation

Uncontrolled Chain Reactions The Atomic Bomb

Little Boy Bomb • The atomic bomb dropped on Hiroshima on August 6, 1945 • Little boy was a U-235 gun-type bomb • Between 80, 000 and 140, 000 people were killed instantly

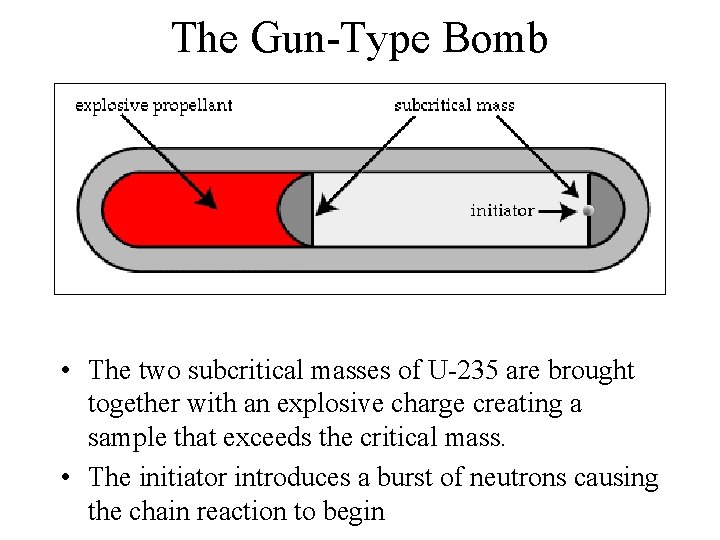
The Gun-Type Bomb • The two subcritical masses of U-235 are brought together with an explosive charge creating a sample that exceeds the critical mass. • The initiator introduces a burst of neutrons causing the chain reaction to begin

Fat Man • A plutonium implosion-type bomb • Dropped on Nagasaki on August 9, 1945 • 74, 000 were killed and 75, 000 severely injured

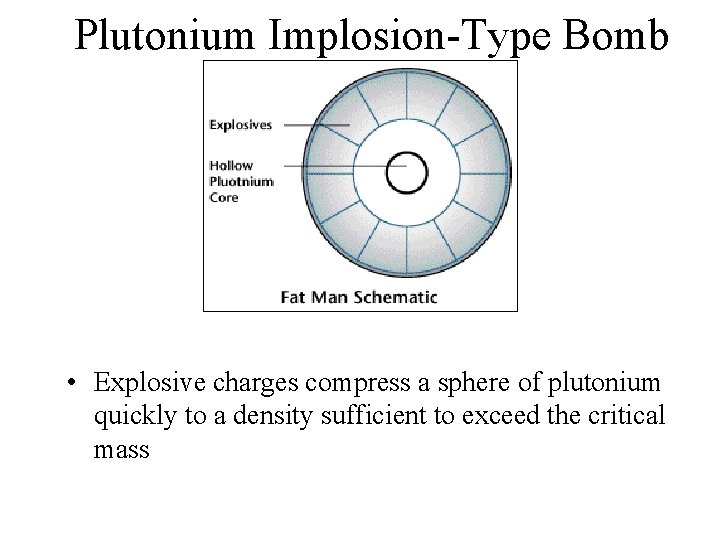
Plutonium Implosion-Type Bomb • Explosive charges compress a sphere of plutonium quickly to a density sufficient to exceed the critical mass

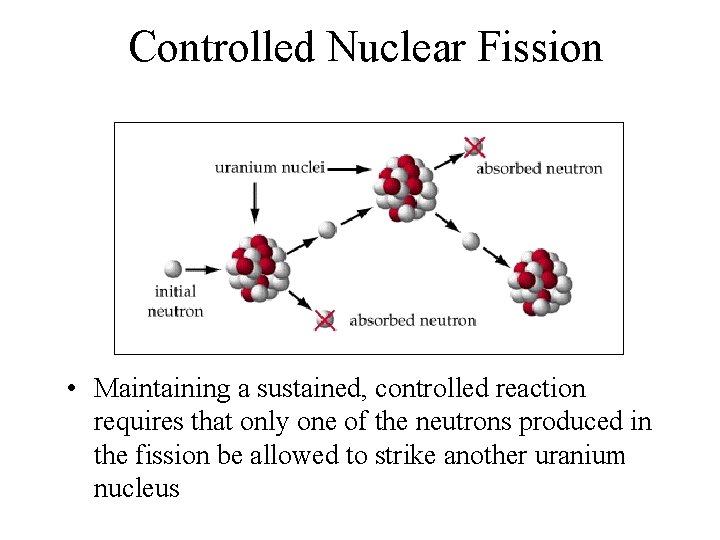
Controlled Nuclear Fission • Maintaining a sustained, controlled reaction requires that only one of the neutrons produced in the fission be allowed to strike another uranium nucleus

Controlled Nuclear Fission • If the ratio of produced neutrons to used neutrons is less than one, the reaction will not be sustained. • If this ratio is greater than one, the reaction will become uncontrolled resulting in an explosion. • A neutron-absorbing material such as graphite can be used to control the chain reaction.

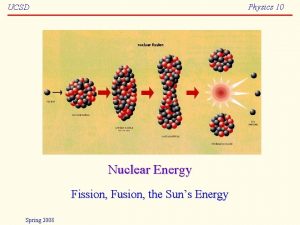
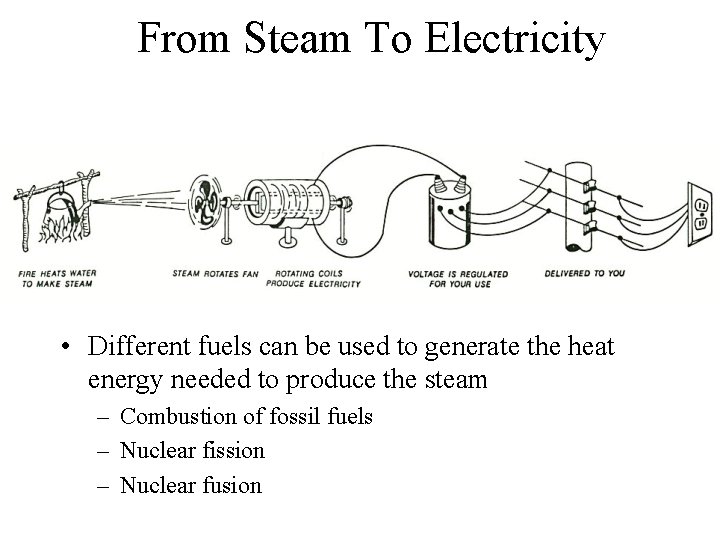
From Steam To Electricity • Different fuels can be used to generate the heat energy needed to produce the steam – Combustion of fossil fuels – Nuclear fission – Nuclear fusion


Types of Fission Reactors • Light Water Reactors (LWR) – Pressurized-light water reactors (PWR) – Boiling water reactors (BWR) • Breeder reactors

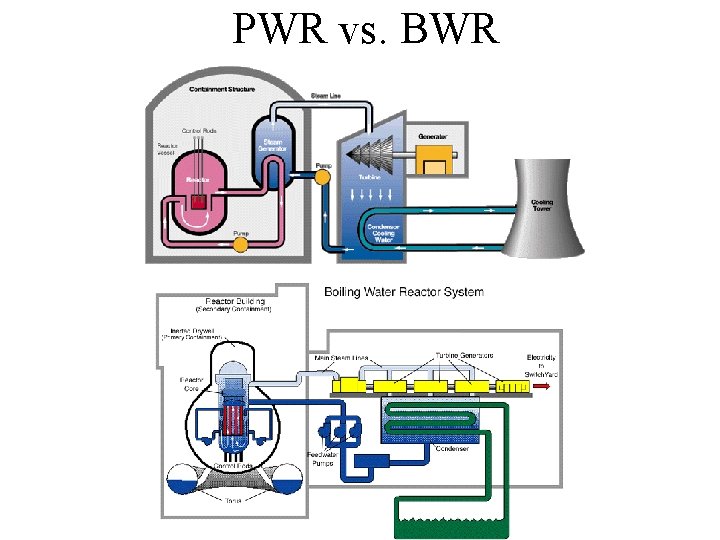
Light Water Reactors • Most popular reactors in U. S. • Use normal water as a coolant and moderator

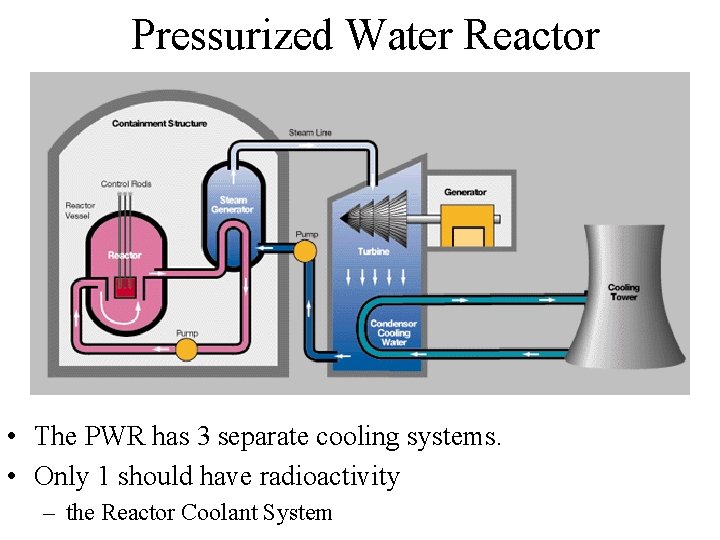
Pressurized Water Reactor • The PWR has 3 separate cooling systems. • Only 1 should have radioactivity – the Reactor Coolant System

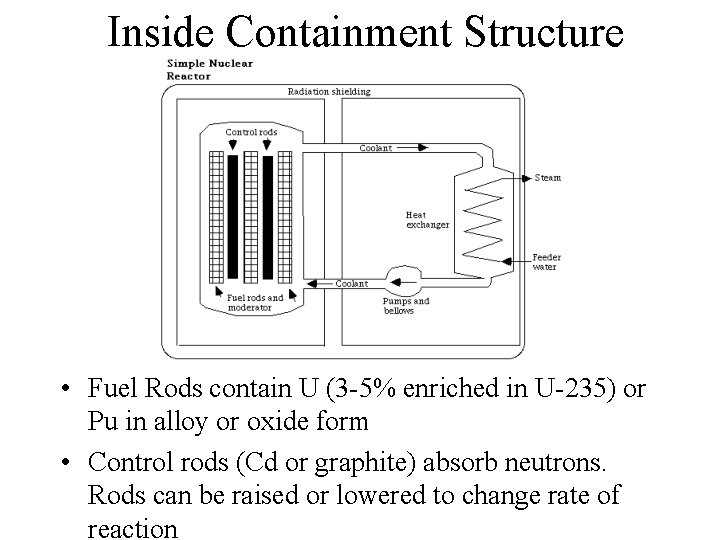
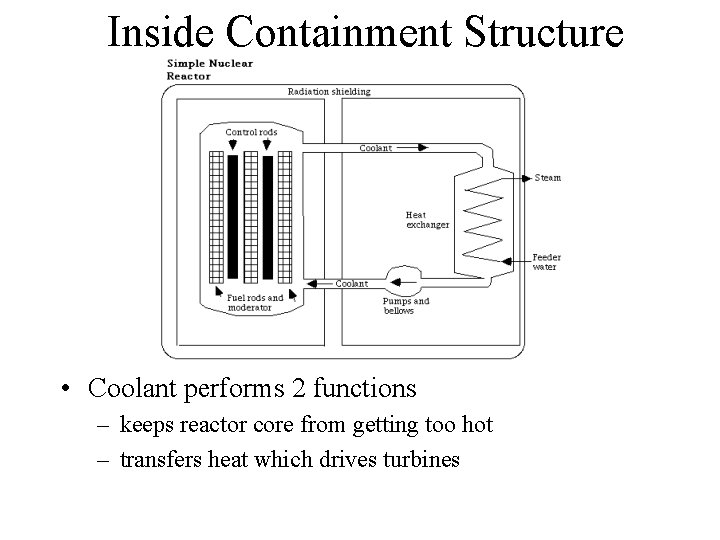
Inside Containment Structure • Fuel Rods contain U (3 -5% enriched in U-235) or Pu in alloy or oxide form • Control rods (Cd or graphite) absorb neutrons. Rods can be raised or lowered to change rate of reaction


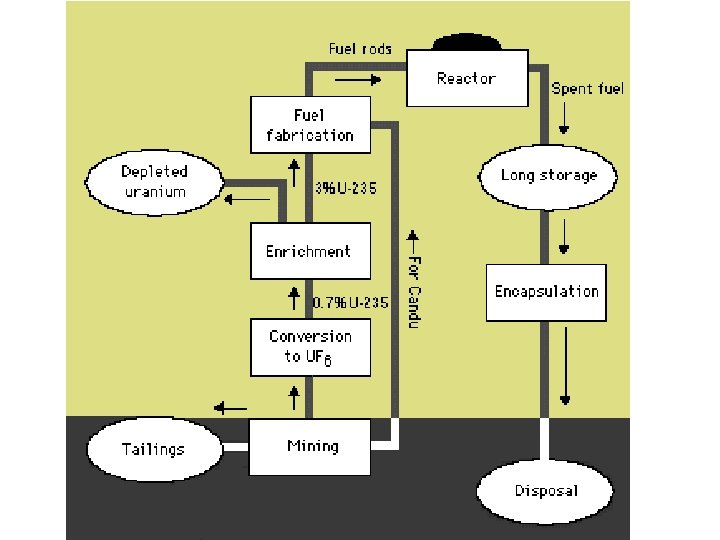
U-235 Enrichment • Naturally occurring uranium is 99. 3% non fissile U-238 & 0. 7% fissile U-235 • In light water reactors, this amount of U-235 is not sufficient to sustain the chain reaction • The uranium is processed to increase the amount of U-235 in the mixture

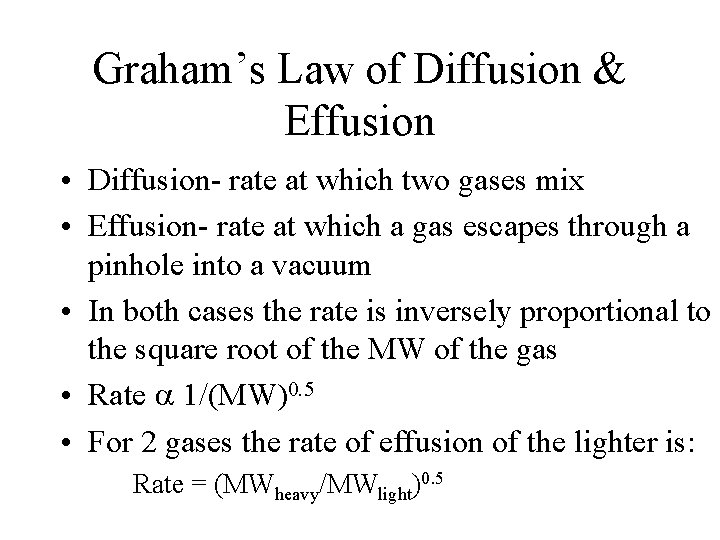
Graham’s Law of Diffusion & Effusion • Diffusion- rate at which two gases mix • Effusion- rate at which a gas escapes through a pinhole into a vacuum • In both cases the rate is inversely proportional to the square root of the MW of the gas • Rate 1/(MW)0. 5 • For 2 gases the rate of effusion of the lighter is: Rate = (MWheavy/MWlight)0. 5

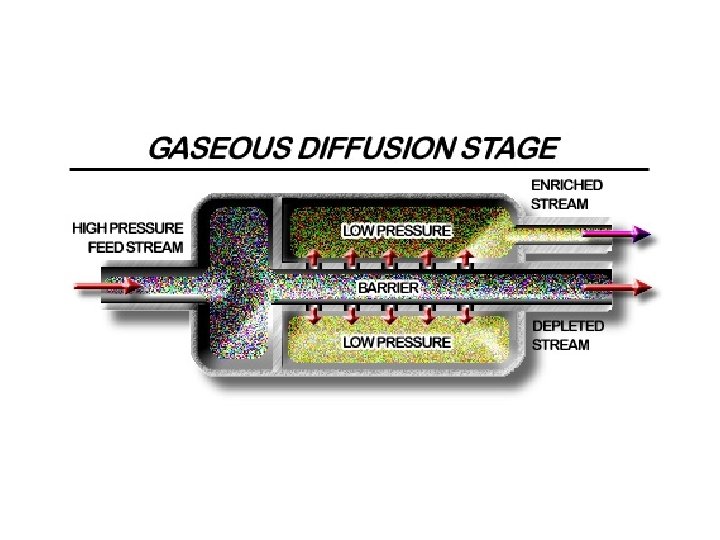
U-235 Enrichment • Rate U-235/238= (352/349)0. 5= 1. 004 • The enrichment after n diffusion barriers is (1. 004)n • Minimum amount of U-235 needed in LWR is 2. 1% (3 x more concentrated than in natural form) • (1. 004)263=3 or 263 diffusion stages needed • Enrichment is an expensive process • Large amount of energy is needed to push the UF 6 through so many barriers


Inside Containment Structure • Coolant performs 2 functions – keeps reactor core from getting too hot – transfers heat which drives turbines

Water as Coolant • Light Water Reactor (LWR) – uses ordinary water – needs enriched uranium fuel – common in U. S. – 80% of world’s reactors • Heavy Water Reactor (HWR) – uses D 2 O – can use natural uranium – common in Canada and Great Britain – 10% of world’s reactors

Water As Coolant • Pressurized Water Reactors – uses a heat exchanger – keeps water that passes the reactor core in a closed loop – steam in turbines never touches fuel rods • Boiling Water Reactors – no heat exchanger – water from reactor core goes to turbines – simpler design/greater contamination risk

PWR vs. BWR

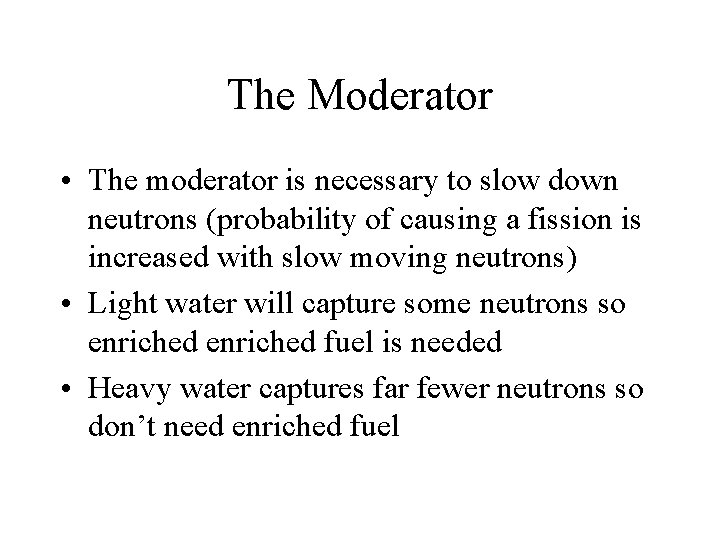
The Moderator • The moderator is necessary to slow down neutrons (probability of causing a fission is increased with slow moving neutrons) • Light water will capture some neutrons so enriched fuel is needed • Heavy water captures far fewer neutrons so don’t need enriched fuel

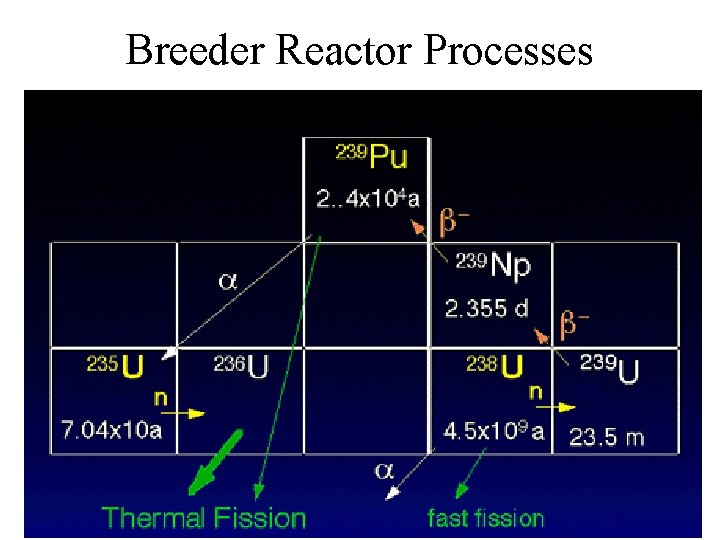
Breeder Reactors • Generate more fissionable material than they consume • Fuel U-238, U-235 & P-239 • No moderator is used – Fast neutrons are captured by non fissionable U -238 to produce U-239 – U-239 decays to fissile Pu-239 • Coolant is liquid sodium metal • None in U. S. – France, Great Britain, Russia

Breeder Reactor Processes

Breeder Reactors • Advantages – creates fissionable material by transforming U 238 into Pu-239 – Fuel less costly

Breeder Reactors • Disadvantages – no moderator (if something goes wrong, it will happen quicker) – liquid sodium coolant is extremely corrosive and dangerous – Plutonium has a critical mass 50% less than uranium • more widely used for weapons • more actively sought by terrorists – Fuel rods require periodic reprocessing to remove contaminants resulting from nuclear reactions (cost consideration)
 Nuclear fission formula
Nuclear fission formula Fission vs fusion nuclear
Fission vs fusion nuclear Artificial transmutation worksheet
Artificial transmutation worksheet Nuclear fission
Nuclear fission Fission equation
Fission equation Nuclear fission
Nuclear fission Nuclear fission explanation
Nuclear fission explanation Nuclear fission and fusion
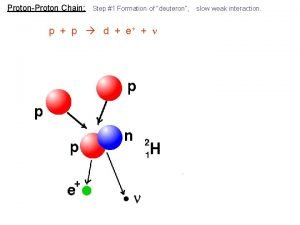
Nuclear fission and fusion Proton proton chain
Proton proton chain Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Nuclear fission
Nuclear fission Nuclear fission and fusion webquest
Nuclear fission and fusion webquest Nuclear fission lise meitner
Nuclear fission lise meitner Geiger counter
Geiger counter Sun fusion or fission
Sun fusion or fission Nuclear fission radiation
Nuclear fission radiation Who discovered radioactivity
Who discovered radioactivity Nuclear fission vs fusion venn diagram
Nuclear fission vs fusion venn diagram Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Uranium atomic mass
Uranium atomic mass The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Perkembangan model atom dalton
Perkembangan model atom dalton Bud a very large man of mass 130 kg
Bud a very large man of mass 130 kg Large scale rotating air mass
Large scale rotating air mass An air mass is created when a large body of air
An air mass is created when a large body of air Very large land mass
Very large land mass Owasp http request smuggling
Owasp http request smuggling Ticket splitting definition
Ticket splitting definition Shear wave splitting
Shear wave splitting Splitting up summations
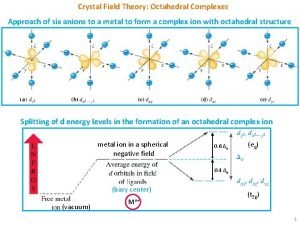
Splitting up summations Crystal field theory for tetrahedral complexes
Crystal field theory for tetrahedral complexes Structure of personality development
Structure of personality development Splitting psykologi
Splitting psykologi Tree vertex splitting
Tree vertex splitting Mehanizmi odbrane primeri
Mehanizmi odbrane primeri Hildegard peplau levels of anxiety
Hildegard peplau levels of anxiety Splitting heart sounds
Splitting heart sounds Identity disturbance
Identity disturbance