Novel Oral Anticoagulant Reversal in Acute Hemorrhage Gretchen



![Warfarin and NOAC Pharmacokinetics Poulsen et al. [2012], Lip et al. [2012] and Perez Warfarin and NOAC Pharmacokinetics Poulsen et al. [2012], Lip et al. [2012] and Perez](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-7.jpg)
![NOAC Monitoring and Reversal Poulsen et al. [2012], Lip et al. [2012] and Perez NOAC Monitoring and Reversal Poulsen et al. [2012], Lip et al. [2012] and Perez](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-12.jpg)


![Perioperative NOAC Discontinuation van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann Perioperative NOAC Discontinuation van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-17.jpg)






- Slides: 35

Novel Oral Anticoagulant Reversal in Acute Hemorrhage Gretchen M. Brophy, Pharm. D, BCPS, FCCP, FCCM, FNCS Professor of Pharmacotherapy & Outcomes Science and Neurosurgery Virginia Commonwealth University Medical College of Virginia Campus

Disclosures • Secretary – Neurocritical Care Society • Research Funding – NIH, Boehringer Ingelheim • Scientific Advisory Board Member – Edge Therapeutics • Consulting/Speaker – Medicines Company, UCB Pharma, Grifols, Cumberland, Mallinckrodt, Bard

Objectives • Discuss anticoagulant agents currently available on the US market. • Identify perioperative considerations for the management of neurotrauma patients on anticoagulant medications. • Describe reversal strategies for neurotrauma patients presenting on anticoagulation medication(s).

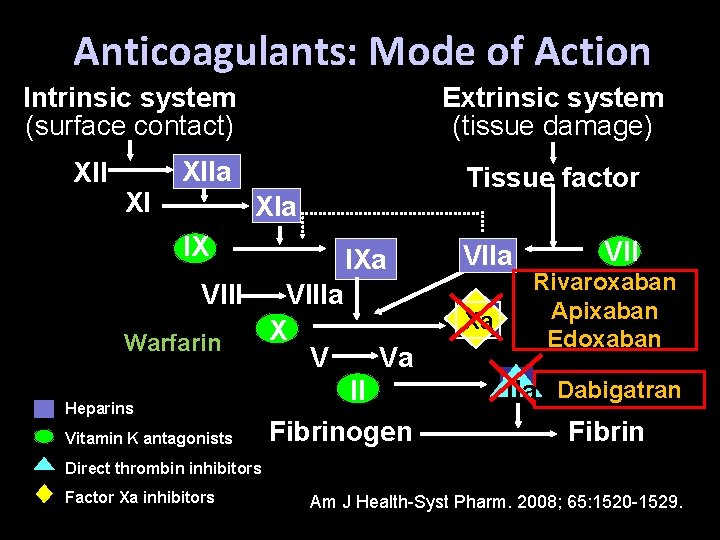
Anticoagulants: Mode of Action Intrinsic system (surface contact) XII XI Extrinsic system (tissue damage) XIIa Tissue factor XIa IX VIII Warfarin Heparins Vitamin K antagonists IXa VIIIa X V VIIa Xa Va II Fibrinogen VII Rivaroxaban Apixaban Edoxaban IIa Dabigatran Fibrin Direct thrombin inhibitors Factor Xa inhibitors Am J Health-Syst Pharm. 2008; 65: 1520 -1529.

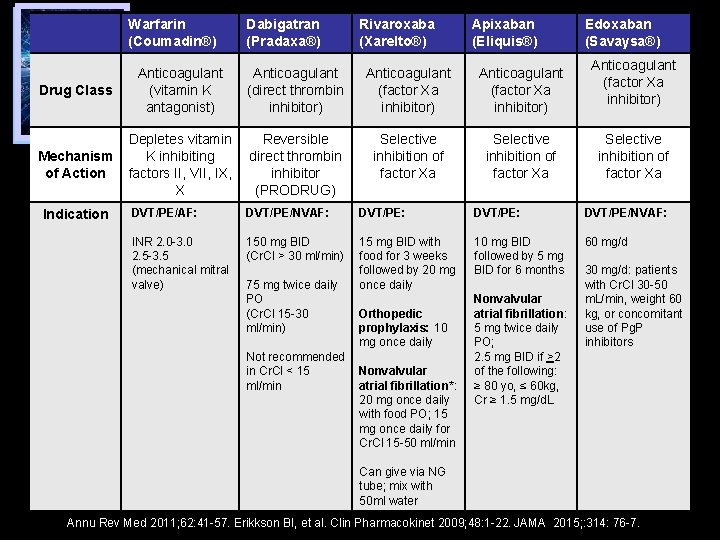
Warfarin (Coumadin®) Drug Class Mechanism of Action Indication Anticoagulant (vitamin K antagonist) Dabigatran (Pradaxa®) Anticoagulant (direct thrombin inhibitor) Reversible Depletes vitamin direct thrombin K inhibiting inhibitor factors II, VII, IX, (PRODRUG) X Rivaroxaba (Xarelto®) Apixaban (Eliquis®) Anticoagulant (factor Xa inhibitor) Selective inhibition of factor Xa Edoxaban (Savaysa®) Anticoagulant (factor Xa inhibitor) Selective inhibition of factor Xa DVT/PE/AF: DVT/PE/NVAF: DVT/PE: DVT/PE/NVAF: INR 2. 0 -3. 0 2. 5 -3. 5 (mechanical mitral valve) 150 mg BID (Cr. Cl > 30 ml/min) 15 mg BID with food for 3 weeks followed by 20 mg once daily 10 mg BID followed by 5 mg BID for 6 months 60 mg/d 75 mg twice daily PO (Cr. Cl 15 -30 ml/min) Orthopedic prophylaxis: 10 mg once daily Not recommended in Cr. Cl < 15 Nonvalvular ml/min atrial fibrillation*: 20 mg once daily with food PO; 15 mg once daily for Cr. Cl 15 -50 ml/min Nonvalvular atrial fibrillation: 5 mg twice daily PO; 2. 5 mg BID if >2 of the following: ≥ 80 yo, ≤ 60 kg, Cr ≥ 1. 5 mg/d. L 30 mg/d: patients with Cr. Cl 30 -50 m. L/min, weight 60 kg, or concomitant use of Pg. P inhibitors Can give via NG tube; mix with 50 ml water Annu Rev Med 2011; 62: 41 -57. Erikkson BI, et al. Clin Pharmacokinet 2009; 48: 1 -22. JAMA 2015; : 314: 76 -7.

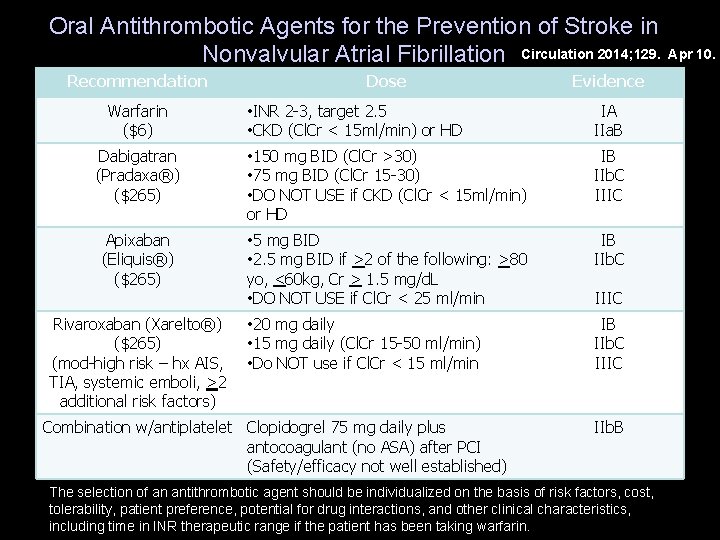
Oral Antithrombotic Agents for the Prevention of Stroke in Nonvalvular Atrial Fibrillation Circulation 2014; 129. Apr 10. Recommendation Warfarin ($6) Dose Evidence • INR 2 -3, target 2. 5 • CKD (Cl. Cr < 15 ml/min) or HD IA IIa. B Dabigatran (Pradaxa®) ($265) • 150 mg BID (Cl. Cr >30) • 75 mg BID (Cl. Cr 15 -30) • DO NOT USE if CKD (Cl. Cr < 15 ml/min) or HD IB IIb. C IIIC Apixaban (Eliquis®) ($265) • 5 mg BID • 2. 5 mg BID if >2 of the following: >80 yo, <60 kg, Cr > 1. 5 mg/d. L • DO NOT USE if Cl. Cr < 25 ml/min IB IIb. C • 20 mg daily • 15 mg daily (Cl. Cr 15 -50 ml/min) • Do NOT use if Cl. Cr < 15 ml/min IB IIb. C IIIC Rivaroxaban (Xarelto®) ($265) (mod-high risk – hx AIS, TIA, systemic emboli, >2 additional risk factors) Combination w/antiplatelet Clopidogrel 75 mg daily plus antocoagulant (no ASA) after PCI (Safety/efficacy not well established) IIIC IIb. B The selection of an antithrombotic agent should be individualized on the basis of risk factors, cost, tolerability, patient preference, potential for drug interactions, and other clinical characteristics, including time in INR therapeutic range if the patient has been taking warfarin.
![Warfarin and NOAC Pharmacokinetics Poulsen et al 2012 Lip et al 2012 and Perez Warfarin and NOAC Pharmacokinetics Poulsen et al. [2012], Lip et al. [2012] and Perez](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-7.jpg)
Warfarin and NOAC Pharmacokinetics Poulsen et al. [2012], Lip et al. [2012] and Perez et al. [2013] Ther Adv in Drug Safe. 2014; 5(1): 8 -20.

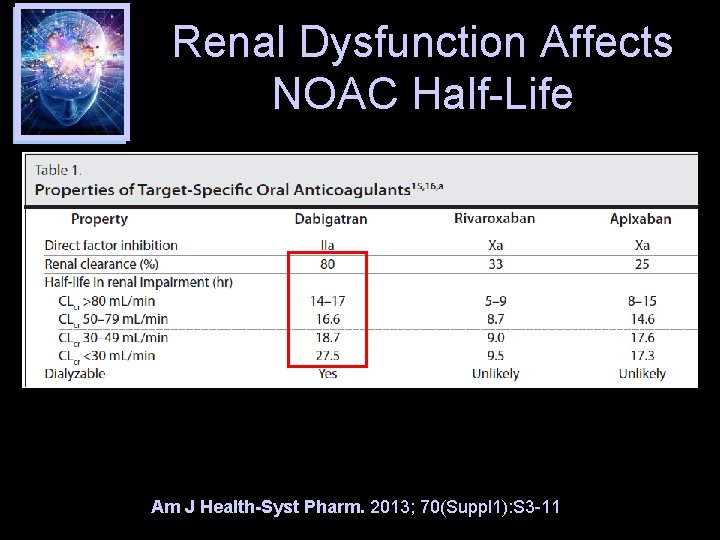
Renal Dysfunction Affects NOAC Half-Life Am J Health-Syst Pharm. 2013; 70(Suppl 1): S 3 -11

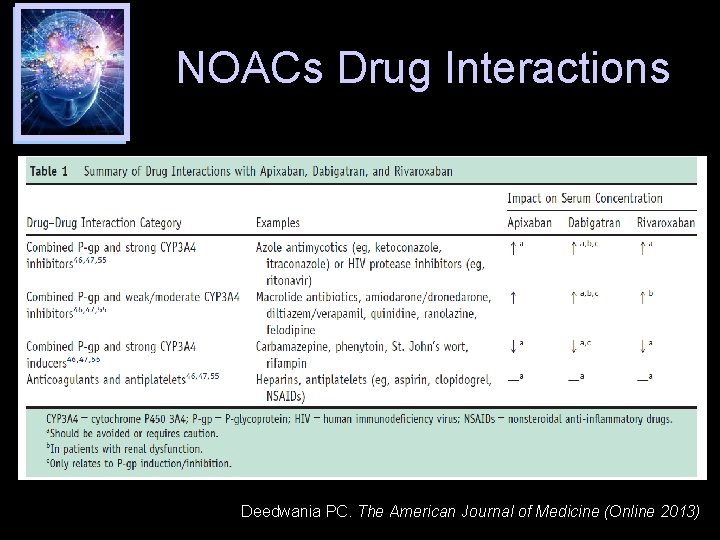
NOACs Drug Interactions Deedwania PC. The American Journal of Medicine (Online 2013)

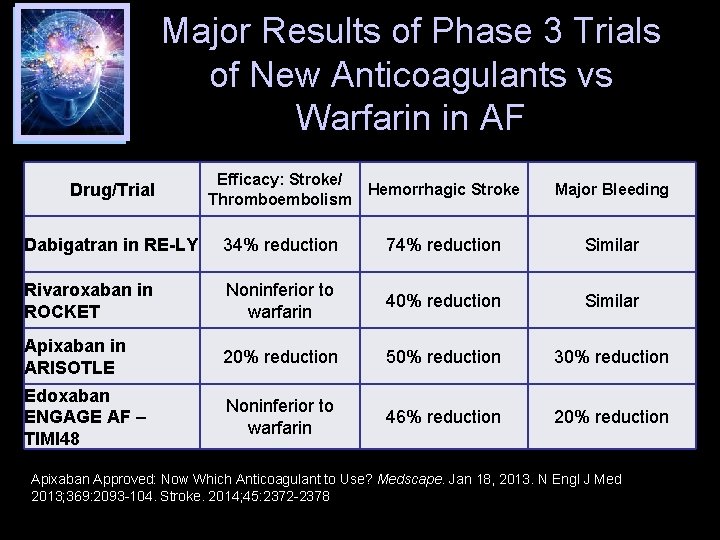
Major Results of Phase 3 Trials of New Anticoagulants vs Warfarin in AF Drug/Trial Efficacy: Stroke/ Hemorrhagic Stroke Thromboembolism Major Bleeding Dabigatran in RE-LY 34% reduction 74% reduction Similar Rivaroxaban in ROCKET Noninferior to warfarin 40% reduction Similar Apixaban in ARISOTLE 20% reduction 50% reduction 30% reduction Edoxaban ENGAGE AF – TIMI 48 Noninferior to warfarin 46% reduction 20% reduction Apixaban Approved: Now Which Anticoagulant to Use? Medscape. Jan 18, 2013. N Engl J Med 2013; 369: 2093 -104. Stroke. 2014; 45: 2372 -2378

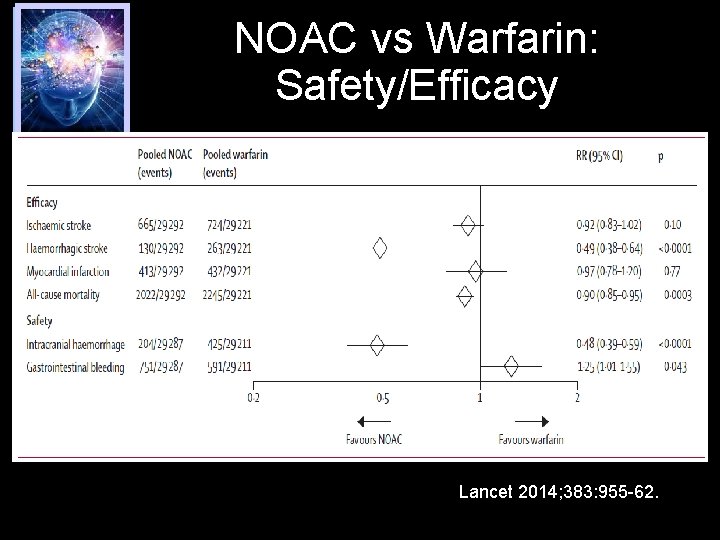
NOAC vs Warfarin: Safety/Efficacy Lancet 2014; 383: 955 -62.
![NOAC Monitoring and Reversal Poulsen et al 2012 Lip et al 2012 and Perez NOAC Monitoring and Reversal Poulsen et al. [2012], Lip et al. [2012] and Perez](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-12.jpg)
NOAC Monitoring and Reversal Poulsen et al. [2012], Lip et al. [2012] and Perez et al. [2013] Ther Adv in Drug Safe. 2014; 5(1): 8 -20.

Case • AP is a 55 yo African American female who presents post MVA with + LOC, a left temporal contusion, GCS 6, and rib fractures. • She has a history of non-valvular Afib, CVA, HTN and diabetes. Wt. 55 kg, BUN 44, Cr is 2. 1. • She is currently taking rivaroxaban 20 mg daily, furosemide 20 mg daily, lisinopril 40 mg daily, and metformin 500 mg BID.

Case • What laboratory monitoring can be done to help determine if this patient is at risk of bleeding from rivaroxaban? A. B. C. D. INR PT a. PTT Anti-Xa activity

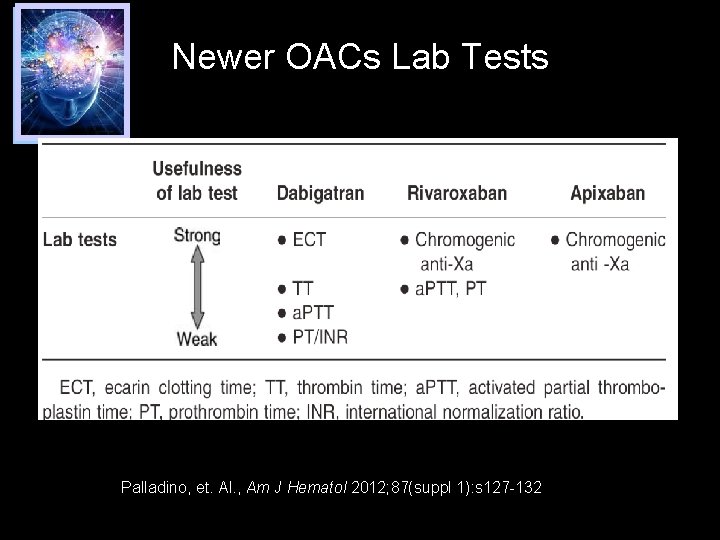
Newer OACs Lab Tests Palladino, et. Al. , Am J Hematol 2012; 87(suppl 1): s 127 -132

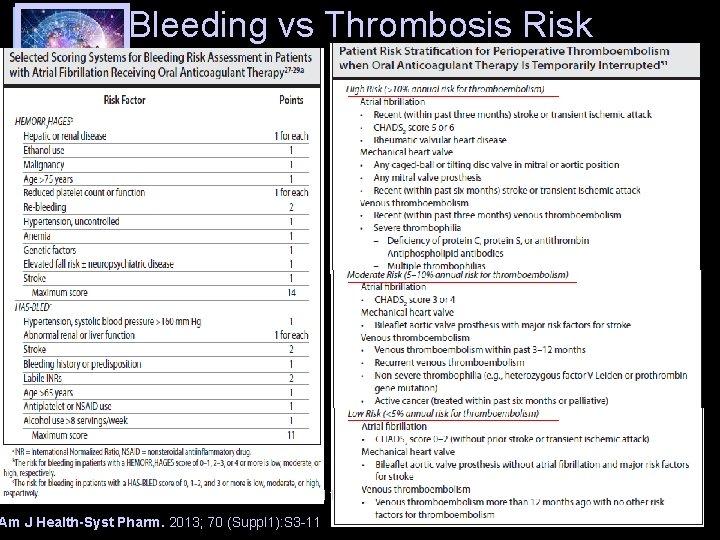
Bleeding vs Thrombosis Risk Am J Health-Syst Pharm. 2013; 70 (Suppl 1): S 3 -11
![Perioperative NOAC Discontinuation van Ryn et al 2010 Spyropoulos and Douketis 2012 and Baumann Perioperative NOAC Discontinuation van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann](https://slidetodoc.com/presentation_image/3b6a0af21993ed833b33897ae4fc35cf/image-17.jpg)
Perioperative NOAC Discontinuation van Ryn et al. [2010], Spyropoulos and Douketis [2012] and Baumann Kreuziger et al. [2012]. Ther Adv in Drug Safe. 2014; 5(1): 8 -20.

Resumption of Therapy • Warfarin therapy should generally be resumed: – 12 - 24 hours after surgery • Unless substantial risk of delayed bleeding or reoperation anticipated • NOAC therapy should generally be resumed: – 24 - 48 hours after a minor procedure – 48 - 72 hours after major surgery • Bridging Therapy: (UFH or LMWH in high risk patients) – NOAC should be resumed • 1 hr before UFH infusion is discontinued or • 10 -12 hours after the last scheduled dose of LMWH Am J Health-Syst Pharm. 2013; 70 (Suppl 1): S 3 -11. J Cardivasc Electrophysiol. 2011(8): 948 -55. N Engl J Med 2013; 368: 2113 -24.

Case • ZZ is a 46 yo construction worker who fell off a roof (about 30 ft) at 11 AM this morning and has a TBI (IPH with midline shift). His GCS is 3. He needs emergent neurosurgery. • PMH: DVT 4 weeks ago • Scr = 2. 3 , Clcr is 31 ml/min, PT 12, a. PTT 45 • Meds: Family states he was on one of those new blood thinning agents, but not sure which one. What reversal strategy would you recommend?

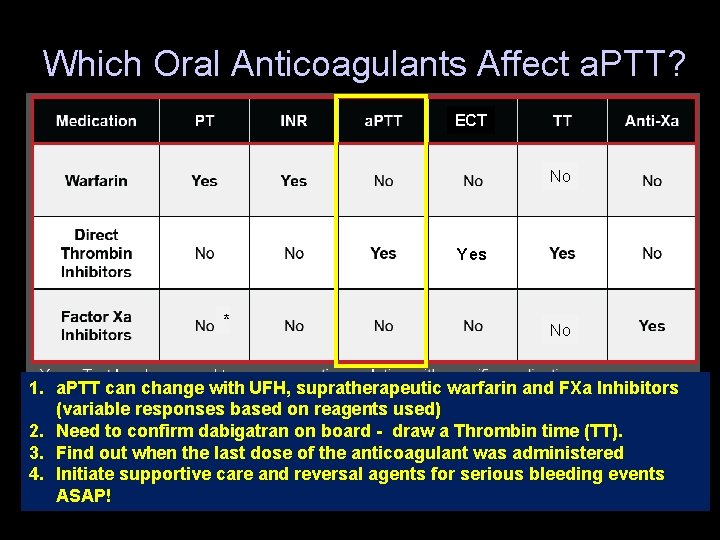
Which Oral Anticoagulants Affect a. PTT? ECT No Yes * No 1. a. PTT can change with UFH, supratherapeutic warfarin and FXa Inhibitors (variable responses based on reagents used) 2. Need to confirm dabigatran on board - draw a Thrombin time (TT). * Rivaroxaban only 3. Find out when the last dose of the anticoagulant was administered 4. Initiate supportive care and reversal agents for serious bleeding events CHEST 2010; 137(1): 184 -94 ASAP!

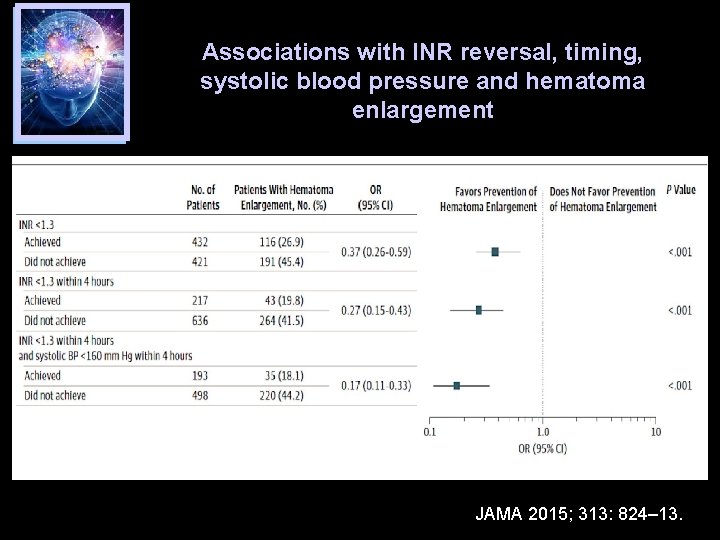
Associations with INR reversal, timing, systolic blood pressure and hematoma enlargement JAMA 2015; 313: 824– 13.

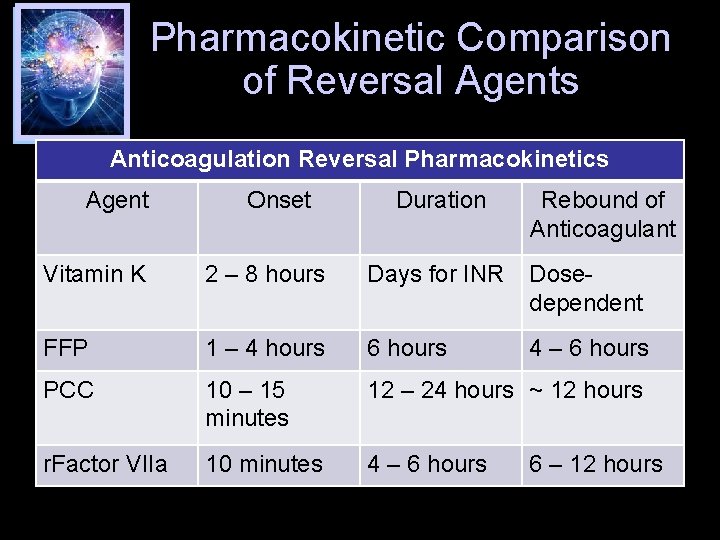
Pharmacokinetic Comparison of Reversal Agents Anticoagulation Reversal Pharmacokinetics Agent Onset Duration Rebound of Anticoagulant Vitamin K 2 – 8 hours Days for INR Dosedependent FFP 1 – 4 hours 6 hours 4 – 6 hours PCC 10 – 15 minutes 12 – 24 hours ~ 12 hours r. Factor VIIa 10 minutes 4 – 6 hours 6 – 12 hours

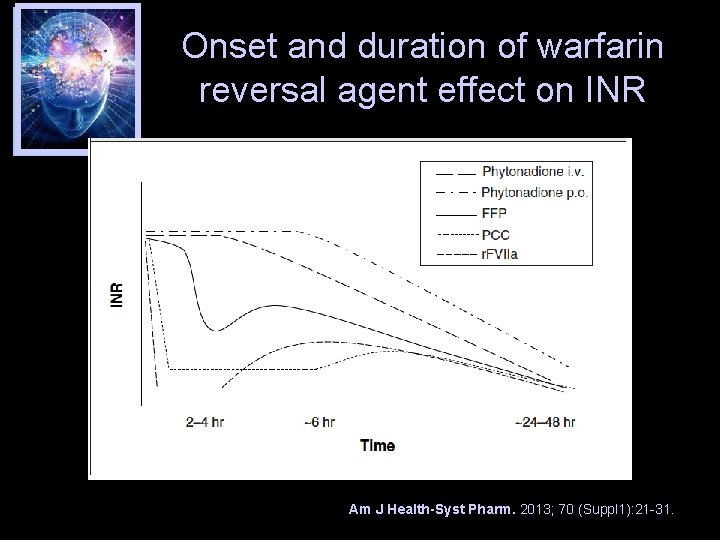
Onset and duration of warfarin reversal agent effect on INR Am J Health-Syst Pharm. 2013; 70 (Suppl 1): 21 -31.

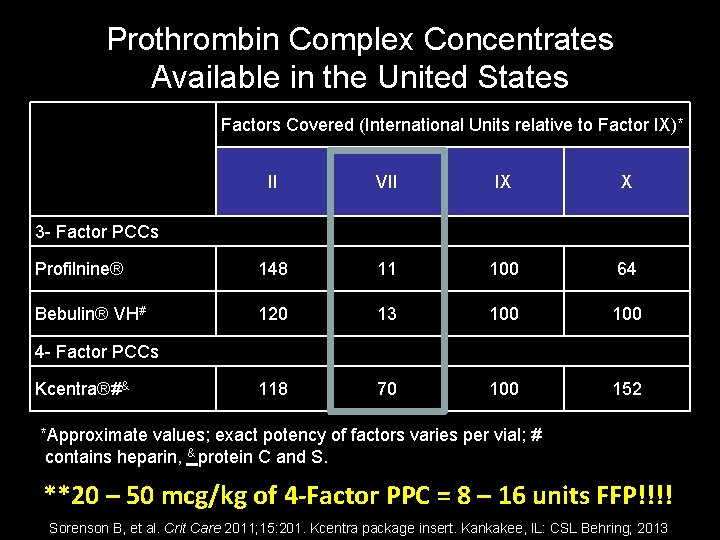
Prothrombin Complex Concentrates Available in the United States Factors Covered (International Units relative to Factor IX)* II VII IX X Profilnine® 148 11 100 64 Bebulin® VH# 120 13 100 118 70 100 152 3 - Factor PCCs 4 - Factor PCCs Kcentra®#& *Approximate values; exact potency of factors varies per vial; # contains heparin, & protein C and S. **20 – 50 mcg/kg of 4 -Factor PPC = 8 – 16 units FFP!!!! Sorenson B, et al. Crit Care 2011; 15: 201. Kcentra package insert. Kankakee, IL: CSL Behring; 2013

Kcentra Dosing: Warfarin Reversal (FDA approved) Ø Dosed on actual body weight up to 100 kg Ø Max rate of IV administration: 8. 4 ml/min (210 U/min)

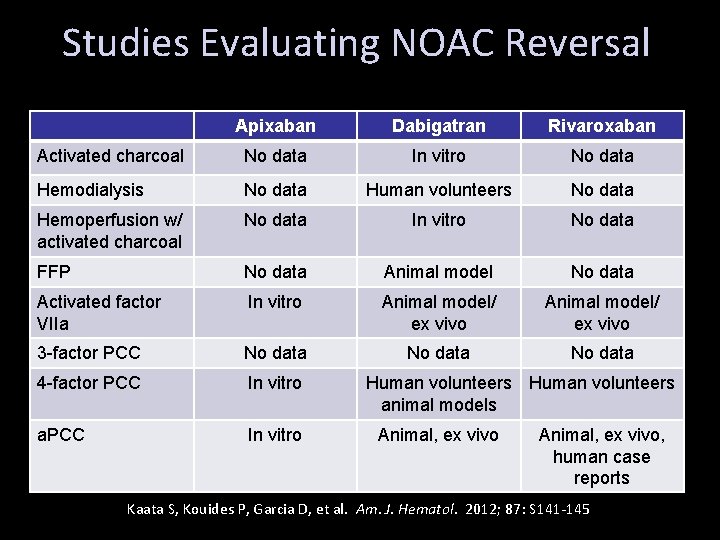
Studies Evaluating NOAC Reversal Apixaban Dabigatran Rivaroxaban Activated charcoal No data In vitro No data Hemodialysis No data Human volunteers No data Hemoperfusion w/ activated charcoal No data In vitro No data FFP No data Animal model No data Activated factor VIIa In vitro Animal model/ ex vivo 3 -factor PCC No data 4 -factor PCC In vitro a. PCC In vitro Human volunteers animal models Animal, ex vivo, human case reports Kaata S, Kouides P, Garcia D, et al. Am. J. Hematol. 2012; 87: S 141 -145

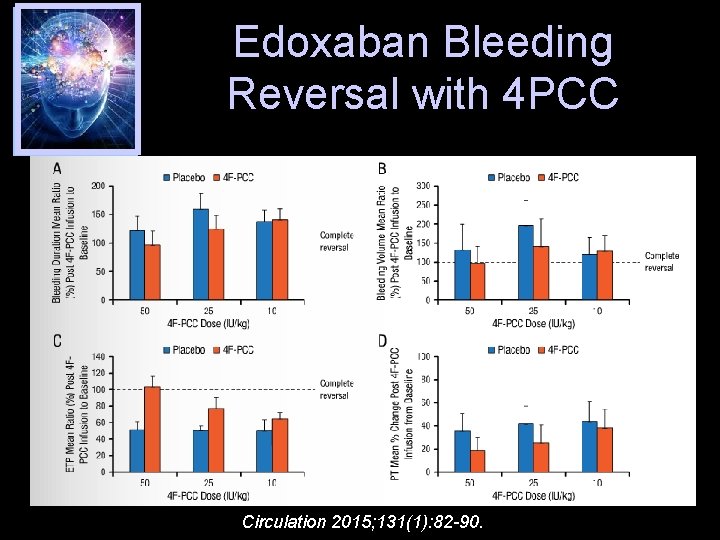
Edoxaban Bleeding Reversal with 4 PCC Circulation 2015; 131(1): 82 -90.

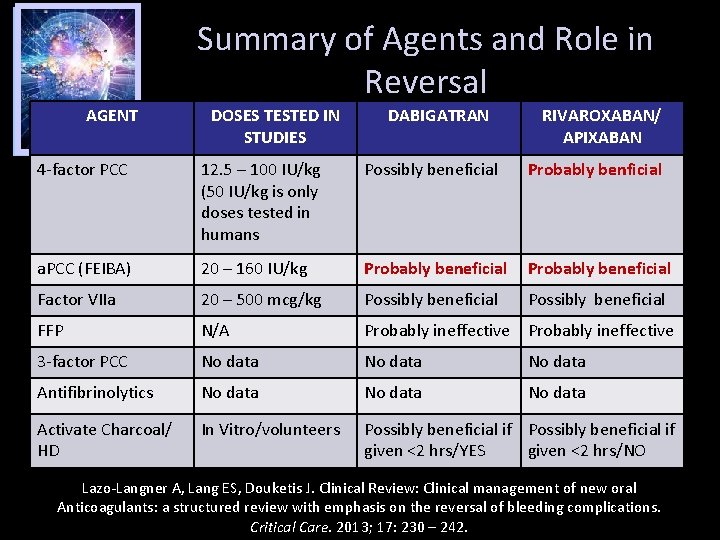
Summary of Agents and Role in Reversal AGENT DOSES TESTED IN STUDIES DABIGATRAN RIVAROXABAN/ APIXABAN 4 -factor PCC 12. 5 – 100 IU/kg (50 IU/kg is only doses tested in humans Possibly beneficial Probably benficial a. PCC (FEIBA) 20 – 160 IU/kg Probably beneficial Factor VIIa 20 – 500 mcg/kg Possibly beneficial FFP N/A Probably ineffective 3 -factor PCC No data Antifibrinolytics No data Activate Charcoal/ HD In Vitro/volunteers Possibly beneficial if given <2 hrs/YES given <2 hrs/NO Lazo-Langner A, Lang ES, Douketis J. Clinical Review: Clinical management of new oral Anticoagulants: a structured review with emphasis on the reversal of bleeding complications. Critical Care. 2013; 17: 230 – 242.

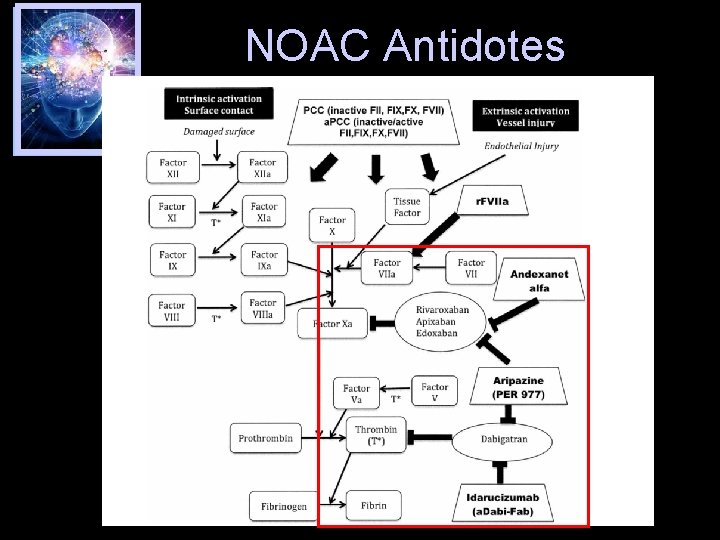
NOAC Antidotes

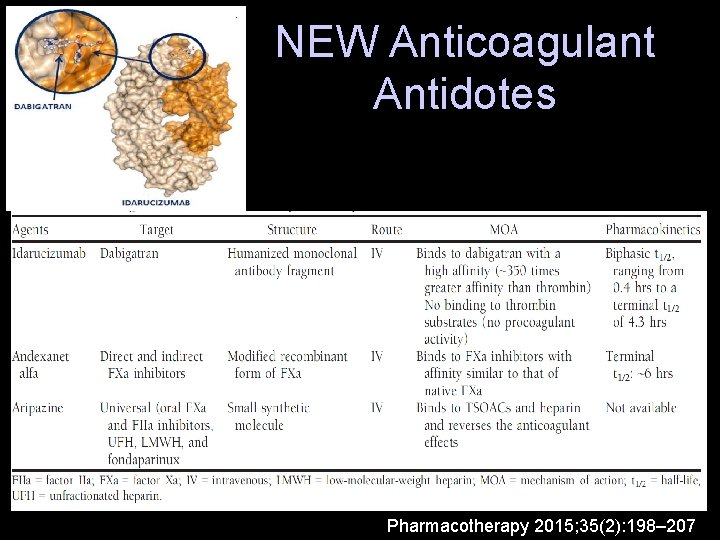
NEW Anticoagulant Antidotes Pharmacotherapy 2015; 35(2): 198– 207

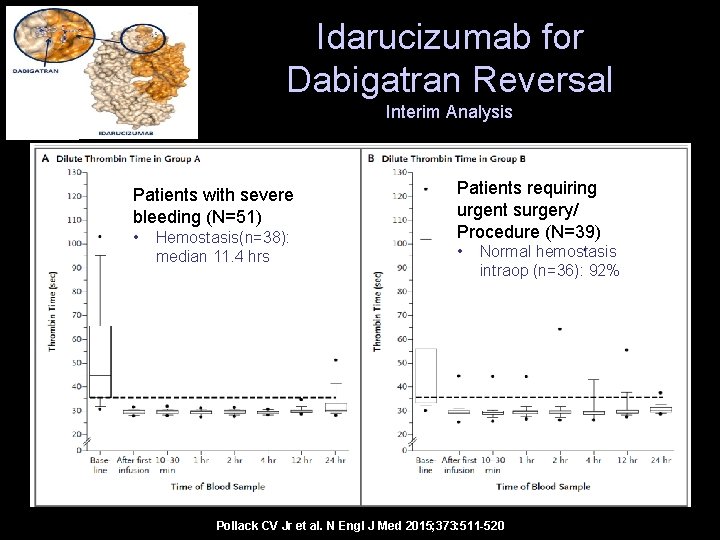
Idarucizumab for Dabigatran Reversal Interim Analysis Patients with severe bleeding (N=51) • Hemostasis(n=38): median 11. 4 hrs Patients requiring urgent surgery/ Procedure (N=39) • Normal hemostasis intraop (n=36): 92% Pollack CV Jr et al. N Engl J Med 2015; 373: 511 -520

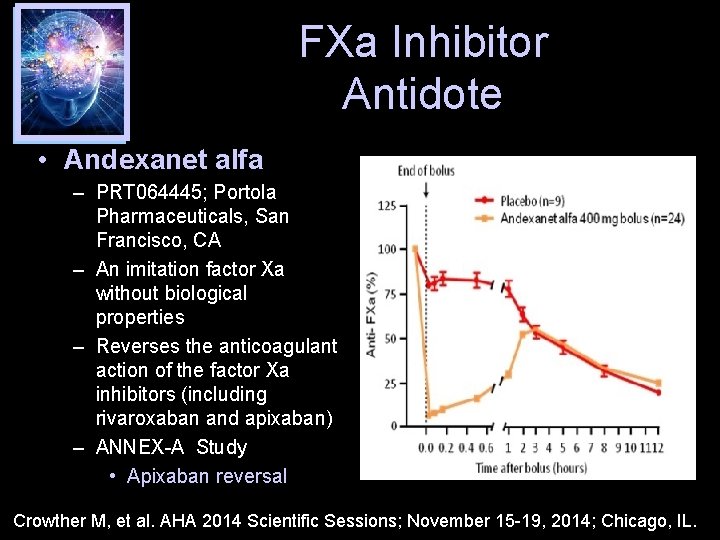
FXa Inhibitor Antidote • Andexanet alfa – PRT 064445; Portola Pharmaceuticals, San Francisco, CA – An imitation factor Xa without biological properties – Reverses the anticoagulant action of the factor Xa inhibitors (including rivaroxaban and apixaban) – ANNEX-A Study • Apixaban reversal Crowther M, et al. AHA 2014 Scientific Sessions; November 15 -19, 2014; Chicago, IL.

Case Ø ZZ is a 46 yo construction worker who fell off a roof (about 30 ft) at 11 AM this morning and has a TBI (IPH with midline shift). His GCS is 3. He needs emergent neurosurgery. Ø Scr = 2. 3 , Clcr is 31 ml/min, PT 12, a. PTT 45 Ø Meds: Dabigatran 150 mg BID Ø What is the best recommendation for reversal of dabigatran for ZZ? A. FFP B. Vitamin K C. PCC D. Hemodialysis

Coming in October 2015 Neurocritical Care Society Guidelines: REVERSAL OF COAGULOPATHY IN INTRACRANIAL HEMORRHAGE

Join the Neurocritical Care Society NCS Membership Benefits – Neurocritical Care journal subscription, including electronic access – Discounted Annual Meeting registration rates – Access to the NCS Discussion Forum – Networking & educational opportunities To join the NCS and begin receiving these benefits immediately, visit our website at www. neurocriticalcare. org. Questions? Email our Executive Office at info@neurocriticalcare. org or call (952) 646 -2031.
 Anticoagulant drugs
Anticoagulant drugs Anticoagulant examples
Anticoagulant examples Warfarin strengths and colors
Warfarin strengths and colors Heparin anticoagulant
Heparin anticoagulant Anticoagulant
Anticoagulant Jean marie connors
Jean marie connors Blood red color code
Blood red color code Anticoagulant
Anticoagulant Thrombolytic vs anticoagulant
Thrombolytic vs anticoagulant Bernardsville middle school
Bernardsville middle school Fe burleson
Fe burleson Gretchen cammiso
Gretchen cammiso Gretchen stanton
Gretchen stanton Faust unterschrift
Faust unterschrift Raymonds run setting
Raymonds run setting Gretchen gueguen
Gretchen gueguen What is the melody of gretchen am spinnrade
What is the melody of gretchen am spinnrade Similarities and differences between squeaky and gretchen
Similarities and differences between squeaky and gretchen Grittylife
Grittylife Gretchen childs
Gretchen childs Gretchen gueguen
Gretchen gueguen Gretchen morgenson
Gretchen morgenson Gretchens entwicklung
Gretchens entwicklung Gretchen childs
Gretchen childs Nursing care plan for postpartum hemorrhage slideshare
Nursing care plan for postpartum hemorrhage slideshare Management of hemorrhage
Management of hemorrhage Types of hemorrhage after tonsillectomy
Types of hemorrhage after tonsillectomy Fetomaternal hemorrhage
Fetomaternal hemorrhage Hemorrhage
Hemorrhage Antipartum hemorrhage
Antipartum hemorrhage Lochia smell
Lochia smell Raina flores sex
Raina flores sex Stamca
Stamca Hemorrhage
Hemorrhage Trauma triad of death
Trauma triad of death Maxill com
Maxill com