Nolitaka Ashizawa Kyoto Univ Introduction perylene D 2




- Slides: 33

Nolitaka Ashizawa Kyoto Univ.

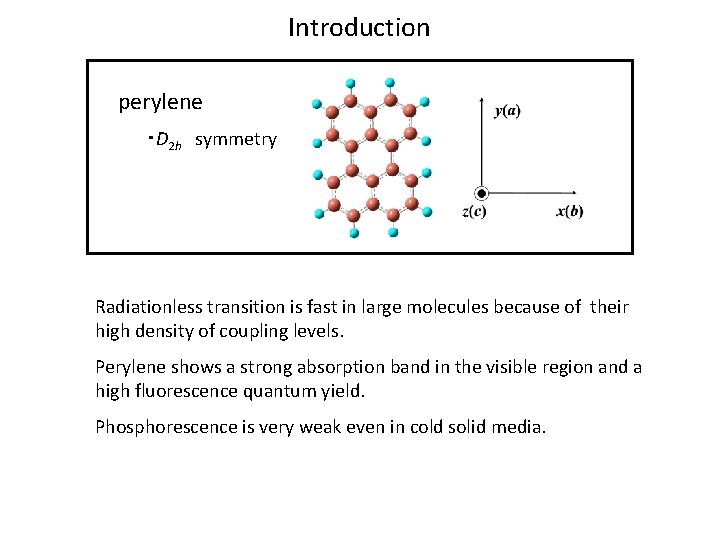
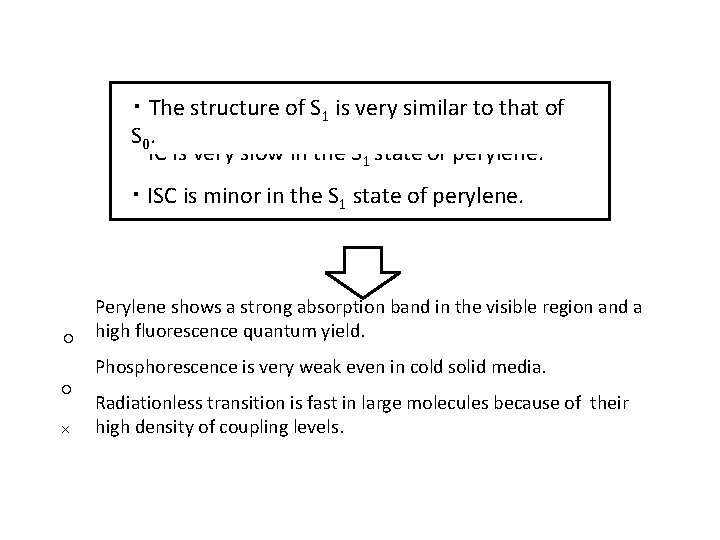
Introduction perylene ・D 2 h symmetry Radiationless transition is fast in large molecules because of their high density of coupling levels. Perylene shows a strong absorption band in the visible region and a high fluorescence quantum yield. Phosphorescence is very weak even in cold solid media.

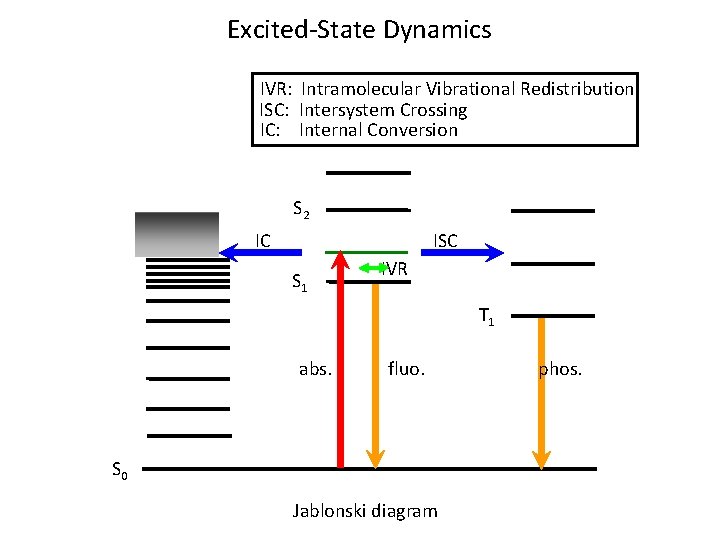
Excited-State Dynamics IVR: Intramolecular Vibrational Redistribution ISC: Intersystem Crossing IC: Internal Conversion S 2 IC ISC S 1 IVR T 1 abs. fluo. S 0 Jablonski diagram phos.

LIF Excitation Spectroscopy Sample + Ar Molecular beam Pulse nozzle Prism PD: Photodiode PM: Photomultiplier PD Chamber PM Prism Dye laser Excimer laser

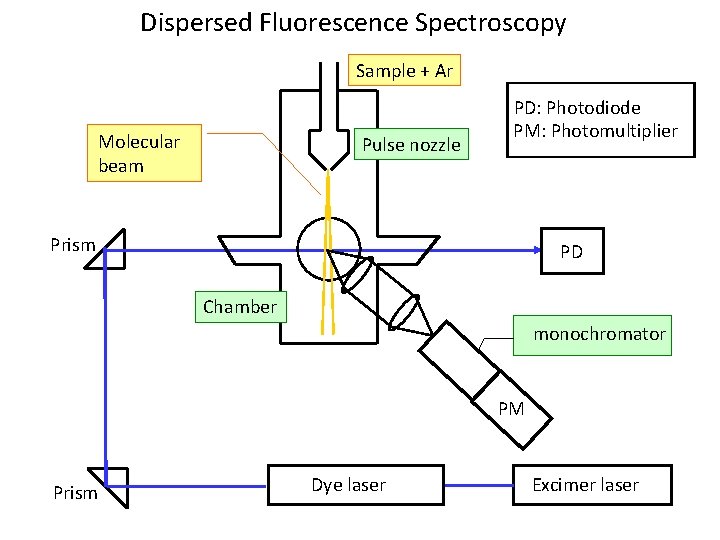
Dispersed Fluorescence Spectroscopy Sample + Ar Molecular beam Pulse nozzle PD: Photodiode PM: Photomultiplier Prism PD Chamber monochromator PM Prism Dye laser Excimer laser

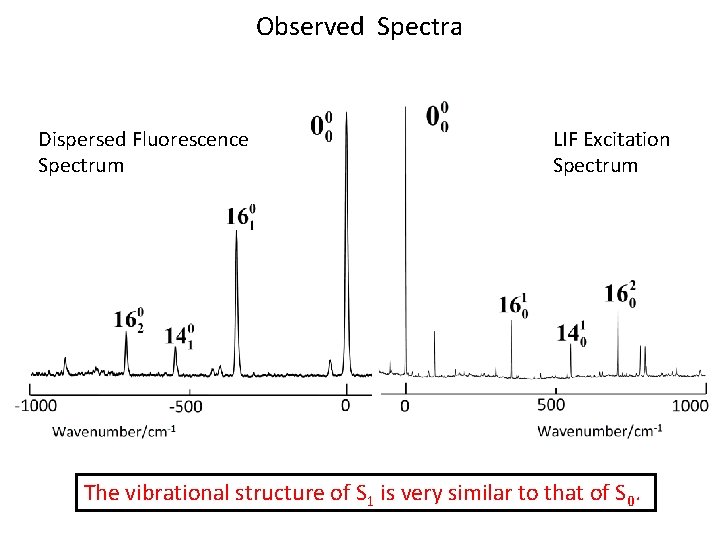
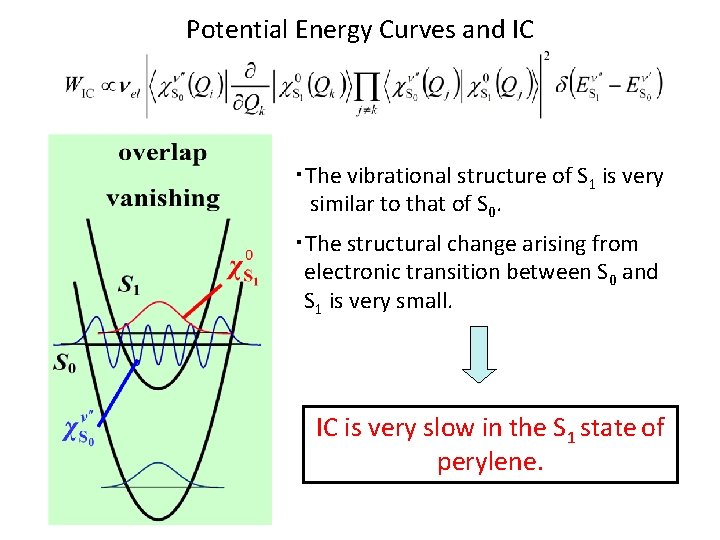
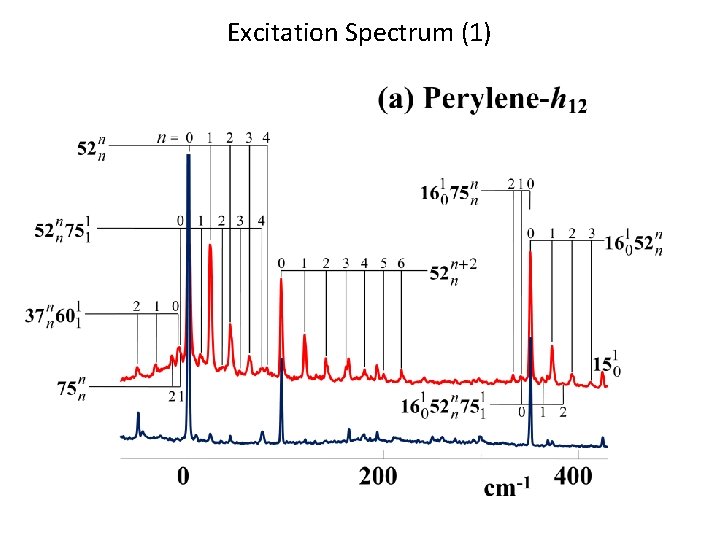
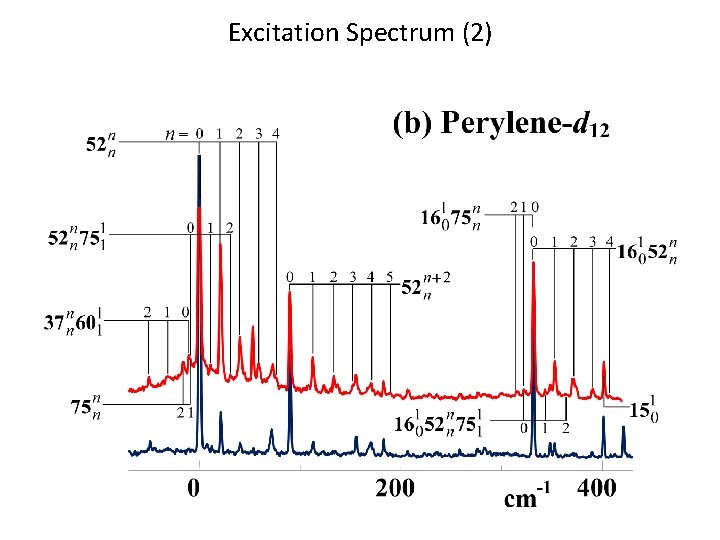
Observed Spectra Dispersed Fluorescence Spectrum LIF Excitation Spectrum The vibrational structure of S 1 is very similar to that of S 0.

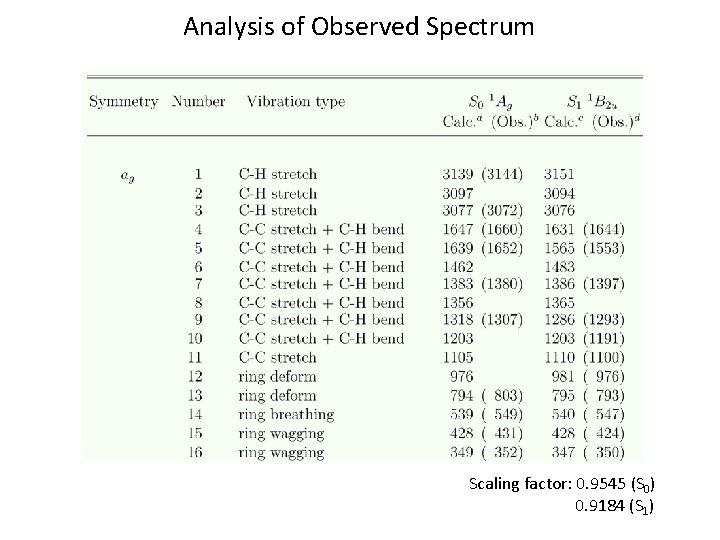
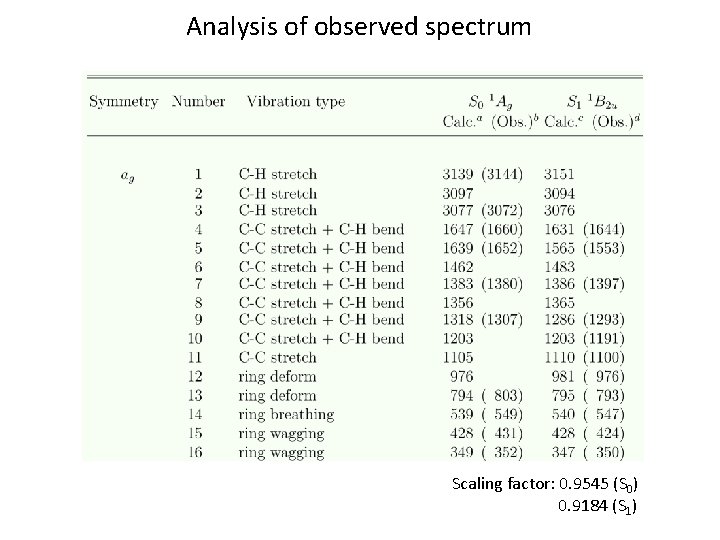
Analysis of Observed Spectrum Scaling factor: 0. 9545 (S 0) 0. 9184 (S 1)

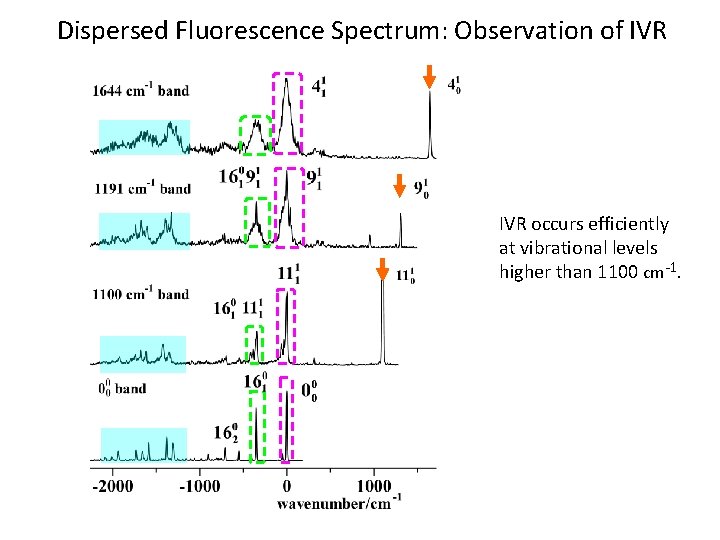
Dispersed Fluorescence Spectrum: Observation of IVR occurs efficiently at vibrational levels higher than 1100 cm -1.

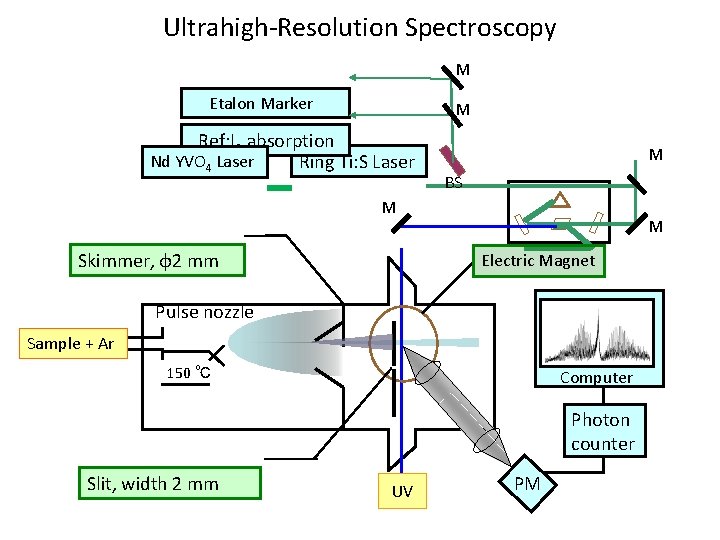
Ultrahigh-Resolution Spectroscopy M Etalon Marker M Ref: I 2 absorption Nd YVO 4 Laser Ring Ti: S Laser M BS M Skimmer, φ2 mm M Electric Magnet Pulse nozzle Sample + Ar 150 ℃ Computer Photon counter Slit, width 2 mm UV PM

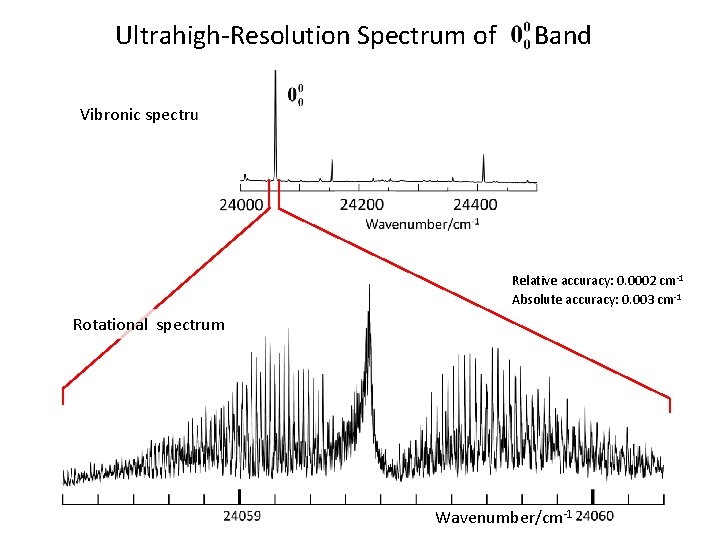
Ultrahigh-Resolution Spectrum of Band Vibronic spectrum Relative accuracy: 0. 0002 cm-1 Absolute accuracy: 0. 003 cm-1 Rotational spectrum Wavenumber/cm-1

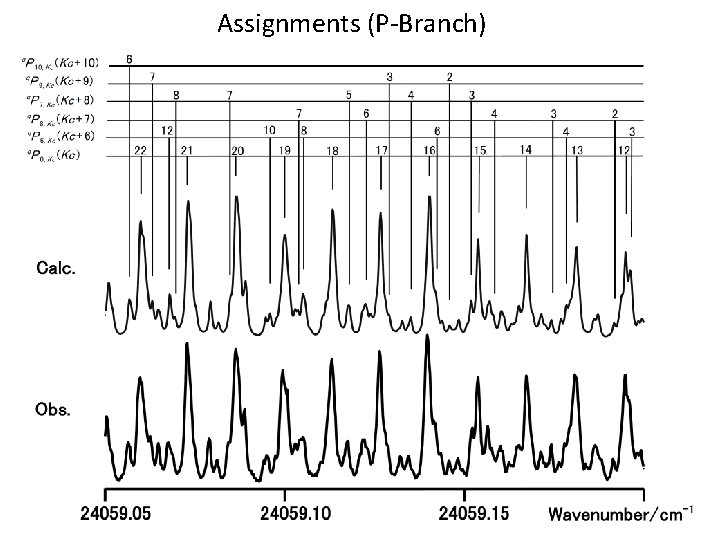
Assignments (P-Branch)

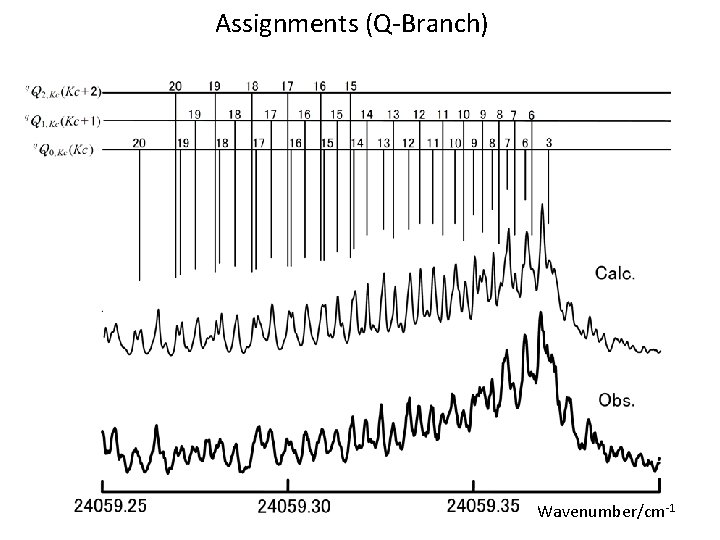
Assignments (Q-Branch) Wavenumber/cm-1

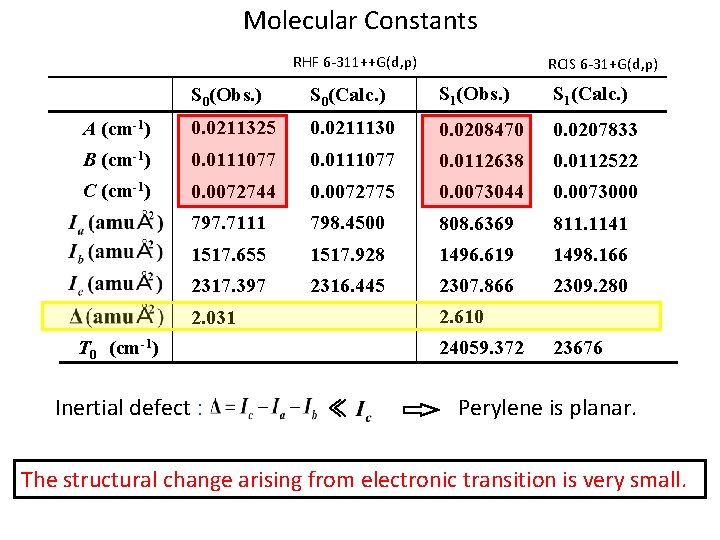
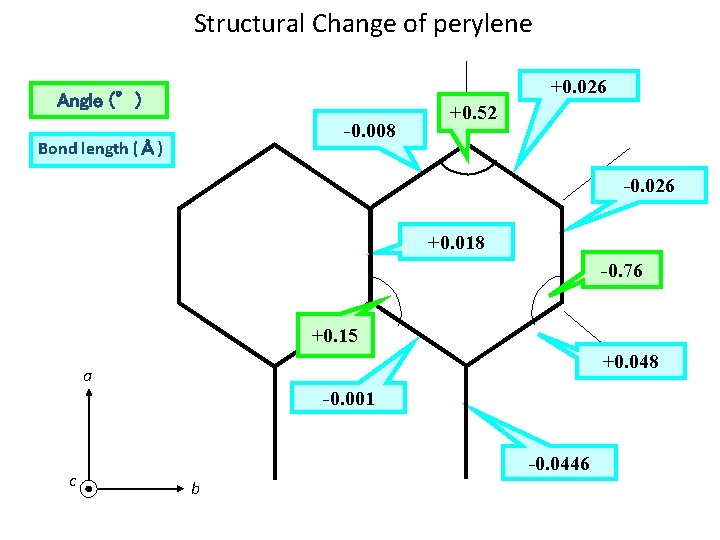
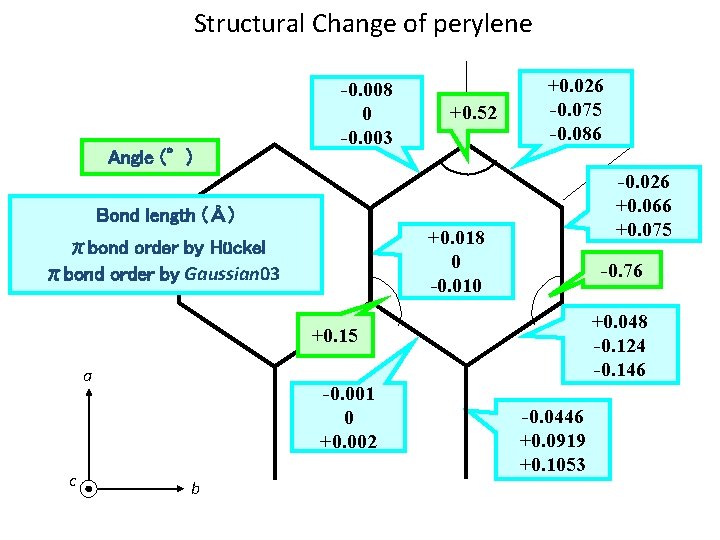
Molecular Constants RHF 6 -311++G(d, p) RCIS 6 -31+G(d, p) S 0(Obs. ) S 0(Calc. ) S 1(Obs. ) S 1(Calc. ) A (cm-1) 0. 0211325 0. 0211130 0. 0208470 0. 0207833 B (cm-1) 0. 0111077 0. 0112638 0. 0112522 C (cm-1) 0. 0072744 0. 0072775 0. 0073044 0. 0073000 797. 7111 798. 4500 808. 6369 811. 1141 1517. 655 1517. 928 1496. 619 1498. 166 2317. 397 2316. 445 2307. 866 2309. 280 2. 610 2. 031 T 0 (cm-1) Inertial defect : 24059. 372 ≪ 23676 Perylene is planar. The structural change arising from electronic transition is very small.

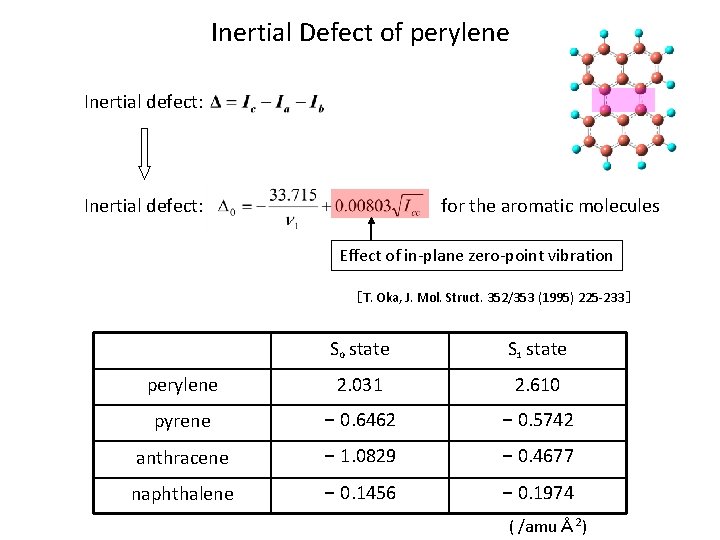
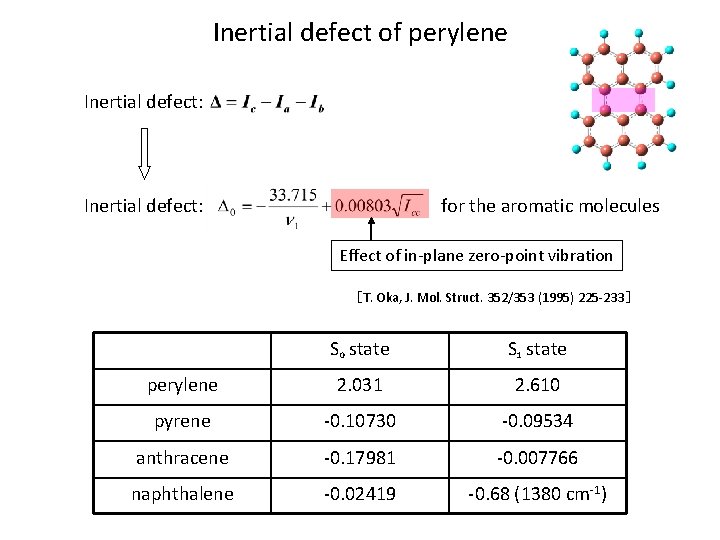
Inertial Defect of perylene Inertial defect: for the aromatic molecules Effect of in-plane zero-point vibration [T. Oka, J. Mol. Struct. 352/353 (1995) 225 -233] S 0 state S 1 state perylene 2. 031 2. 610 pyrene - 0. 6462 - 0. 5742 anthracene - 1. 0829 - 0. 4677 naphthalene - 0. 1456 - 0. 1974 ( /amuÅ 2)

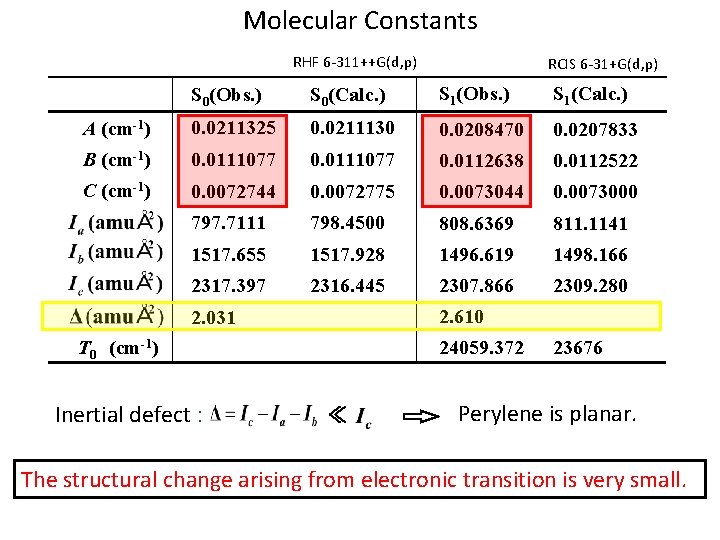
Molecular Constants RHF 6 -311++G(d, p) RCIS 6 -31+G(d, p) S 0(Obs. ) S 0(Calc. ) S 1(Obs. ) S 1(Calc. ) A (cm-1) 0. 0211325 0. 0211130 0. 0208470 0. 0207833 B (cm-1) 0. 0111077 0. 0112638 0. 0112522 C (cm-1) 0. 0072744 0. 0072775 0. 0073044 0. 0073000 797. 7111 798. 4500 808. 6369 811. 1141 1517. 655 1517. 928 1496. 619 1498. 166 2317. 397 2316. 445 2307. 866 2309. 280 2. 610 2. 031 T 0 (cm-1) Inertial defect : 24059. 372 ≪ 23676 Perylene is planar. The structural change arising from electronic transition is very small.

Structural Change of perylene +0. 026 Angle (°) -0. 008 Bond length (Å) +0. 52 -0. 026 +0. 018 -0. 76 +0. 15 +0. 048 a -0. 001 c -0. 0446 b

Analysis of observed spectrum Scaling factor: 0. 9545 (S 0) 0. 9184 (S 1)

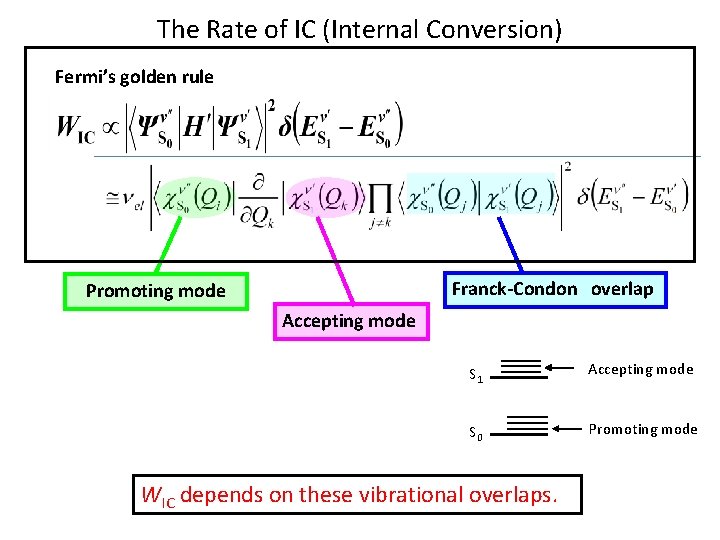
The Rate of IC (Internal Conversion) Fermi’s golden rule Franck-Condon overlap Promoting mode Accepting mode S 1 Accepting mode S 0 Promoting mode WIC depends on these vibrational overlaps.

Potential Energy Curves and IC ・The vibrational structure of S 1 is very similar to that of S 0. ・The structural change arising from electronic transition between S 0 and S 1 is very small. IC is very slow in the S 1 state of perylene.

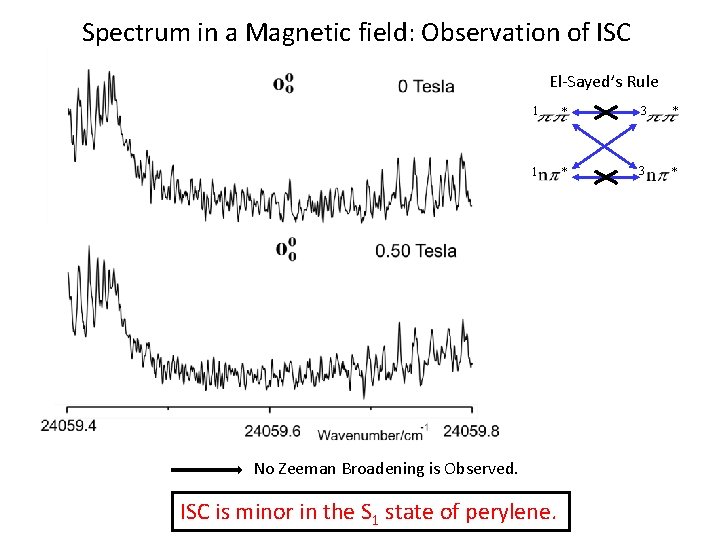
Spectrum in a Magnetic field: Observation of ISC El-Sayed’s Rule 1 * 3 * No Zeeman Broadening is Observed. ISC is minor in the S 1 state of perylene.

Accepting mode and Promoting mode of Internal Conversion

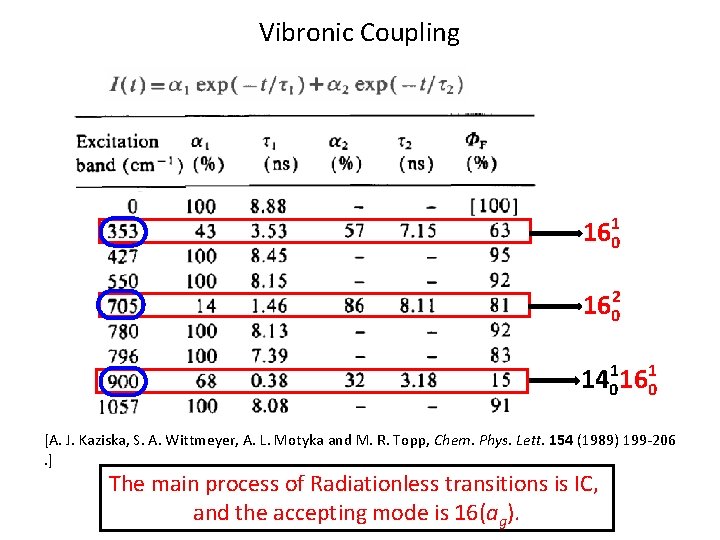
Vibronic Coupling 1601 1602 14011601 [A. J. Kaziska, S. A. Wittmeyer, A. L. Motyka and M. R. Topp, Chem. Phys. Lett. 154 (1989) 199 -206. ] The main process of Radiationless transitions is IC, and the accepting mode is 16(ag).

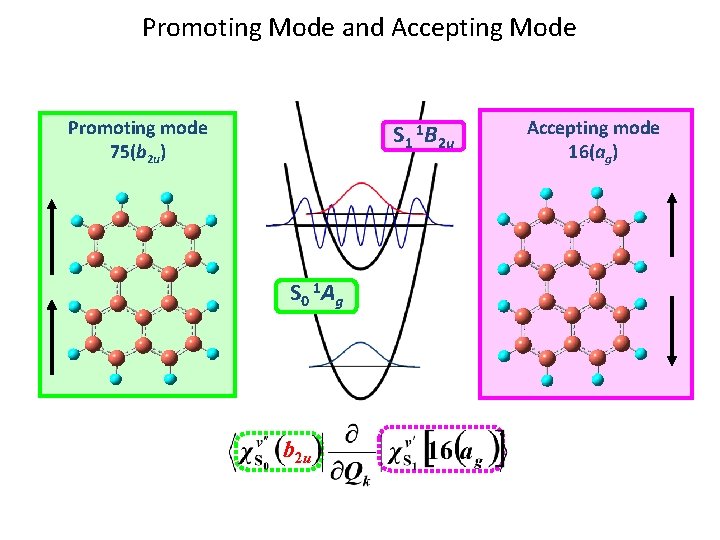
Promoting Mode and Accepting Mode Promoting mode 75(b 2 u) S 1 1 B 2 u S 0 1 A g b 2 u Accepting mode 16(ag)

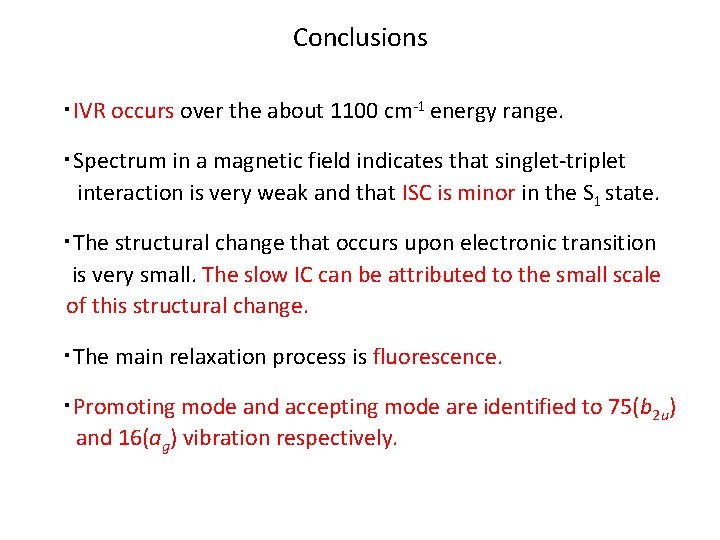
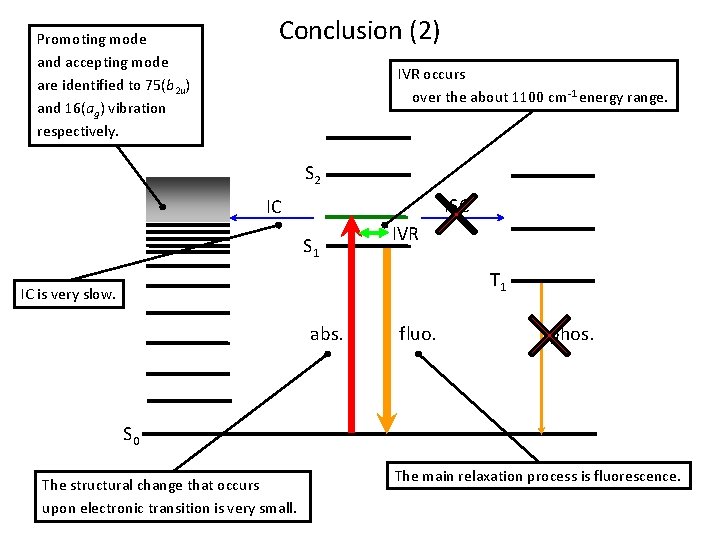
Conclusions ・IVR occurs over the about 1100 cm-1 energy range. ・Spectrum in a magnetic field indicates that singlet-triplet interaction is very weak and that ISC is minor in the S 1 state. ・The structural change that occurs upon electronic transition is very small. The slow IC can be attributed to the small scale of this structural change. ・The main relaxation process is fluorescence. ・Promoting mode and accepting mode are identified to 75(b 2 u) and 16(ag) vibration respectively.

Promoting mode and accepting mode are identified to 75(b 2 u) and 16(ag) vibration respectively. Conclusion (2) IVR occurs over the about 1100 cm-1 energy range. S 2 ISC IC S 1 IVR T 1 IC is very slow. abs. fluo. phos. S 0 The structural change that occurs upon electronic transition is very small. The main relaxation process is fluorescence.

Inertial defect of perylene Inertial defect: for the aromatic molecules Effect of in-plane zero-point vibration [T. Oka, J. Mol. Struct. 352/353 (1995) 225 -233] S 0 state S 1 state perylene 2. 031 2. 610 pyrene -0. 10730 -0. 09534 anthracene -0. 17981 -0. 007766 naphthalene -0. 02419 -0. 68 (1380 cm-1)

Excitation Spectrum (1)

Excitation Spectrum (2)


Structural Change of perylene Angle (°) -0. 008 0 -0. 003 +0. 52 +0. 026 -0. 075 -0. 086 -0. 026 +0. 066 +0. 075 Bond length (Å) +0. 018 0 -0. 010 πbond order by Hückel πbond order by Gaussian 03 -0. 76 +0. 048 -0. 124 -0. 146 +0. 15 a c -0. 001 0 +0. 002 b -0. 0446 +0. 0919 +0. 1053

π Bond Order in S 0 state of perylene πbond order by Hückel Bond length (Å) 0. 7285 0. 13559 0. 5281 0. 14160 0. 6866 0. 14073 0. 4468 0. 14136 0. 6471 0. 13700 a c 0. 5086 0. 14300 b 0. 3887 0. 14855

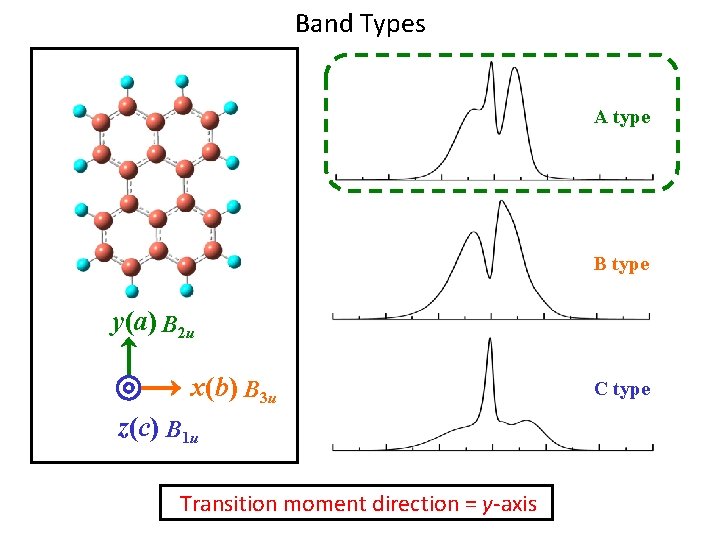
Band Types A type B type y(a) B 2 u x(b) B 3 u z(c) B 1 u Transition moment direction = y-axis C type

・ The structure of S 1 is very similar to that of S 0. ・ IC is very slow in the S 1 state of perylene. ・ ISC is minor in the S 1 state of perylene. ○ ○ × Perylene shows a strong absorption band in the visible region and a high fluorescence quantum yield. Phosphorescence is very weak even in cold solid media. Radiationless transition is fast in large molecules because of their high density of coupling levels.
 Kyoto treaty apush
Kyoto treaty apush Combustivels
Combustivels Kyoto protocol objectives
Kyoto protocol objectives Menguin kyoto
Menguin kyoto Mappa concettuale protocollo di kyoto
Mappa concettuale protocollo di kyoto Kyoto
Kyoto Yitp kyoto
Yitp kyoto Kyoto protocol brazil
Kyoto protocol brazil Prtocolo de kyoto
Prtocolo de kyoto Kyoto protocol
Kyoto protocol Kyoto university vpn
Kyoto university vpn Kyoto protocol reference manual
Kyoto protocol reference manual Kyoto protocol
Kyoto protocol Protocolo quioto
Protocolo quioto Jaehoon yu
Jaehoon yu Medecine dentaire constantine
Medecine dentaire constantine Ch rahmoune
Ch rahmoune Sug grant
Sug grant Fsi umbb
Fsi umbb Scolarite medecine nantes
Scolarite medecine nantes Fs.univ.umbb
Fs.univ.umbb Univ constantine 3
Univ constantine 3 Pharmacie univ batna 2
Pharmacie univ batna 2 Scolarité médecine nantes
Scolarité médecine nantes Université elbayadh
Université elbayadh Prodoc univ nantes
Prodoc univ nantes Univ prof titel
Univ prof titel Univ tln moodle
Univ tln moodle Mail univ ouargla
Mail univ ouargla Ufr sfa poitiers
Ufr sfa poitiers (univ. caxias do sul) escolha a alternativa que completa
(univ. caxias do sul) escolha a alternativa que completa Loncapa ohio university
Loncapa ohio university Ent iut valenciennes
Ent iut valenciennes Ent univ tours
Ent univ tours