New Generation Resolute Integrity DrugEluting Stent Superior to





- Slides: 17

New Generation Resolute Integrity Drug-Eluting Stent Superior to Benchmark Xience Drug. Eluting Stent: Primary Endpoint Results from the PROPEL Study – a Historical Controlled, Prospective, Multicenter, Clinical Study of All -Comers Design Masato Nakamura, MD 1, Satoru Otsuji, MD 2, Yoshihisa Nakagawa, MD 3, Yuji Oikawa, MD 4, Nobuo Shiode, MD 5, Masatoshi Miyahara, MD 6, Gaku Nakazawa, MD 7 and Hiroyoshi Yokoi, MD 8, on behalf of the PROPEL Study Investigators 1 Toho University Medical Center, Ohashi Hospital, Tokyo, Japan; 2 Higashi Takarazuka Satoh Hospital, Hyogo, Japan; 3 Tenri Hospital, Nara, Japan; 4 The Cardiovascular Institute, Tokyo, Japan; 5 Tsuchiya General Hospital, Hiroshima, Japan; 6 Mie Heart Center, Mie, Japan; 7 Tokai University Hospital, Kanagawa, Japan; 8 Fukuoka Sanno Hospital, Fukuoka, Japan ACC 2016 For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

Author Disclosures Dr. Nakamura is a consultant for Terumo Corporation, and lectures for Boston Scientific, Abbott Vascular, Terumo Corporation, and Medtronic Japan. Dr. Nakazawa is a consultant for Abbott Vascular Japan, Terumo Corporation, Japan Medical Device Engineering, and St. Jude Medical, and receives research grants from Abbott Vascular, Terumo Corporation, Boston Scientific, Daiichi Sankyo, and Japan Medical Device Engineering. All other authors have nothing to disclose. For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

Background § The Resolute Integrity™ zotarolimus-eluting stent (Medtronic) utilizes the Continuous Sinusoidal Technology, which forms the stent out of a single wire and improves deliverability. § The PROPEL study is the first large, real world study to assess the safety, efficacy and deliverability of Resolute Integrity compared with a historical control, Xience V™ Everolimus-eluting stent (Abbott Laboratories). For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

Objectives § To assess the safety, efficacy and deliverability of the Resolute Integrity stent in a large real-world Japanese patient population with coronary artery disease implanted with the Resolute Integrity device. § Hypothesis: non-inferiority followed by superiority testing of Resolute Integrity for TLF in patients with only clinical follow-up at 12 months, compared to the Xience arm from R-AC trial as historical control. For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

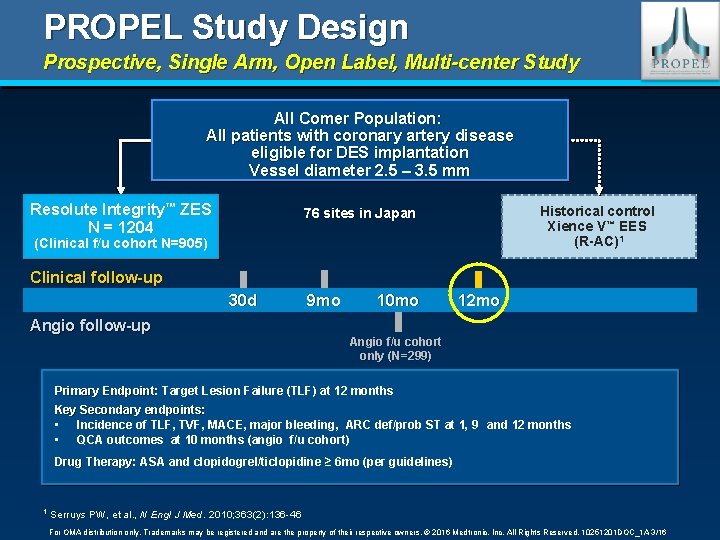
PROPEL Study Design Prospective, Single Arm, Open Label, Multi-center Study All Comer Population: All patients with coronary artery disease eligible for DES implantation Vessel diameter 2. 5 – 3. 5 mm Resolute Integrity™ ZES N = 1204 Historical control Xience V™ EES (R-AC)1 76 sites in Japan (Clinical f/u cohort N=905) Clinical follow-up 30 d Angio follow-up 9 mo 10 mo 12 mo Angio f/u cohort only (N=299) Primary Endpoint: Target Lesion Failure (TLF) at 12 months Key Secondary endpoints: • Incidence of TLF, TVF, MACE, major bleeding, ARC def/prob ST at 1, 9 and 12 months • QCA outcomes at 10 months (angio f/u cohort) Drug Therapy: ASA and clopidogrel/ticlopidine ≥ 6 mo (per guidelines) 1 Serruys PW, et al. , N Engl J Med. 2010; 363(2): 136 -46 For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

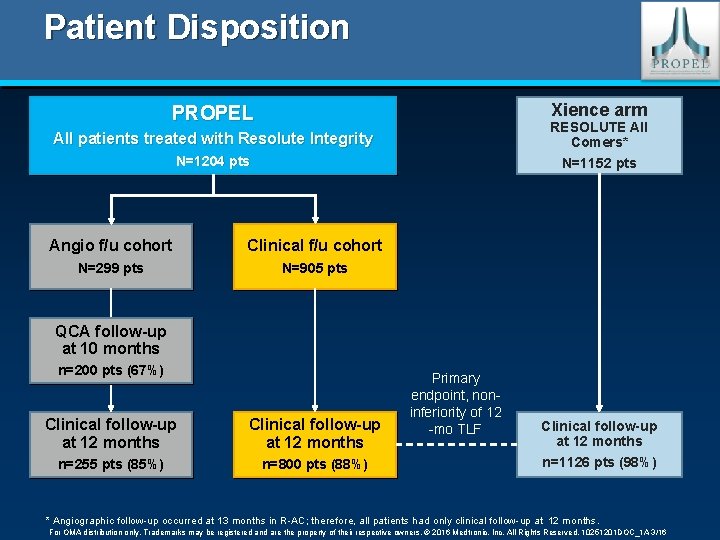
Patient Disposition Xience arm PROPEL All patients treated with Resolute Integrity RESOLUTE All Comers* N=1204 pts N=1152 pts Angio f/u cohort Clinical f/u cohort N=299 pts N=905 pts QCA follow-up at 10 months n=200 pts (67%) Clinical follow-up at 12 months n=255 pts (85%) n=800 pts (88%) Primary endpoint, noninferiority of 12 -mo TLF Clinical follow-up at 12 months n=1126 pts (98%) * Angiographic follow-up occurred at 13 months in R-AC; therefore, all patients had only clinical follow-up at 12 months. For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

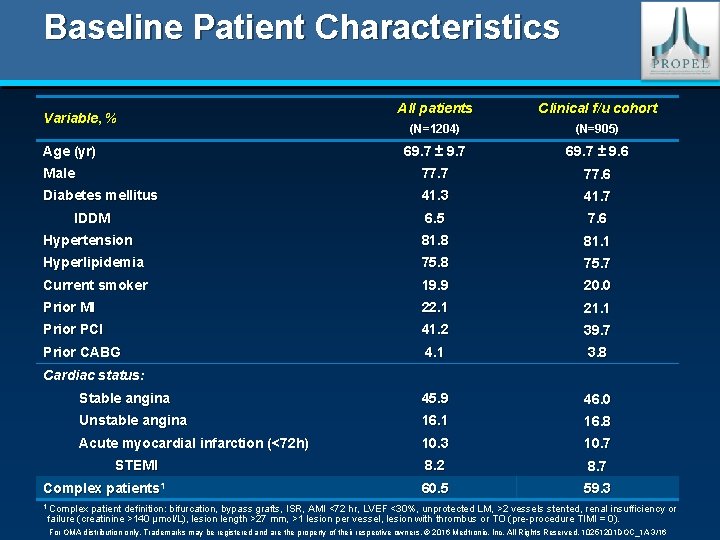
Baseline Patient Characteristics All patients Clinical f/u cohort (N=1204) (N=905) 69. 7 ± 9. 7 69. 7 ± 9. 6 Male 77. 7 77. 6 Diabetes mellitus 41. 3 41. 7 6. 5 7. 6 Hypertension 81. 8 81. 1 Hyperlipidemia 75. 8 75. 7 Current smoker 19. 9 20. 0 Prior MI 22. 1 21. 1 Prior PCI 41. 2 39. 7 Prior CABG 4. 1 3. 8 Stable angina 45. 9 46. 0 Unstable angina 16. 1 16. 8 Acute myocardial infarction (<72 h) 10. 3 10. 7 8. 2 8. 7 60. 5 59. 3 Variable, % Age (yr) IDDM Cardiac status: STEMI Complex patients 1 1 Complex patient definition: bifurcation, bypass grafts, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (pre-procedure TIMI = 0). For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

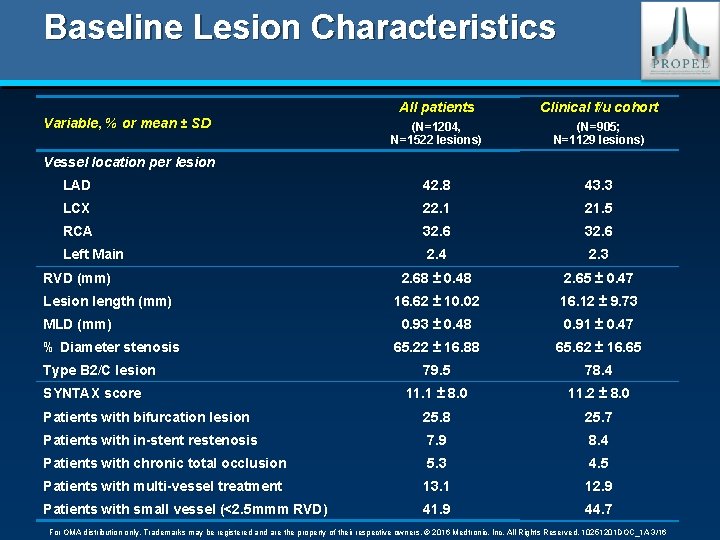
Baseline Lesion Characteristics All patients Clinical f/u cohort (N=1204, N=1522 lesions) (N=905; N=1129 lesions) LAD 42. 8 43. 3 LCX 22. 1 21. 5 RCA 32. 6 Left Main 2. 4 2. 3 2. 68 ± 0. 48 2. 65 ± 0. 47 16. 62 ± 10. 02 16. 12 ± 9. 73 0. 93 ± 0. 48 0. 91 ± 0. 47 65. 22 ± 16. 88 65. 62 ± 16. 65 79. 5 78. 4 11. 1 ± 8. 0 11. 2 ± 8. 0 Patients with bifurcation lesion 25. 8 25. 7 Patients with in-stent restenosis 7. 9 8. 4 Patients with chronic total occlusion 5. 3 4. 5 Patients with multi-vessel treatment 13. 1 12. 9 Patients with small vessel (<2. 5 mmm RVD) 41. 9 44. 7 Variable, % or mean ± SD Vessel location per lesion RVD (mm) Lesion length (mm) MLD (mm) % Diameter stenosis Type B 2/C lesion SYNTAX score For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

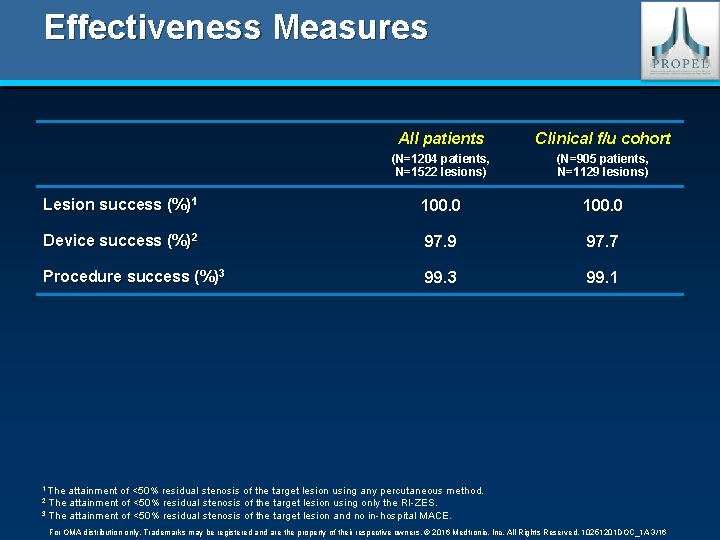
Effectiveness Measures All patients Clinical f/u cohort (N=1204 patients, N=1522 lesions) (N=905 patients, N=1129 lesions) Lesion success (%)1 100. 0 Device success (%)2 97. 9 97. 7 Procedure success (%)3 99. 1 1 The attainment of <50% residual stenosis of the target lesion using any percutaneous method. The attainment of <50% residual stenosis of the target lesion using only the RI-ZES. 3 The attainment of <50% residual stenosis of the target lesion and no in-hospital MACE. 2 For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

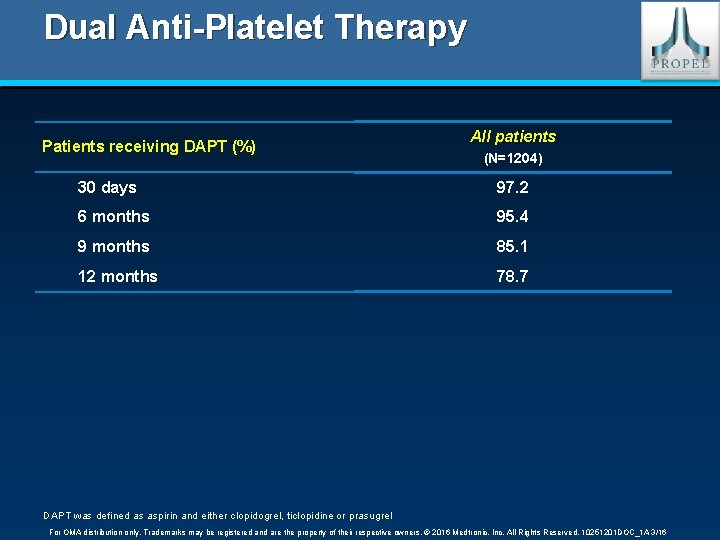
Dual Anti-Platelet Therapy Patients receiving DAPT (%) All patients (N=1204) 30 days 97. 2 6 months 95. 4 9 months 85. 1 12 months 78. 7 DAPT was defined as aspirin and either clopidogrel, ticlopidine or prasugrel For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

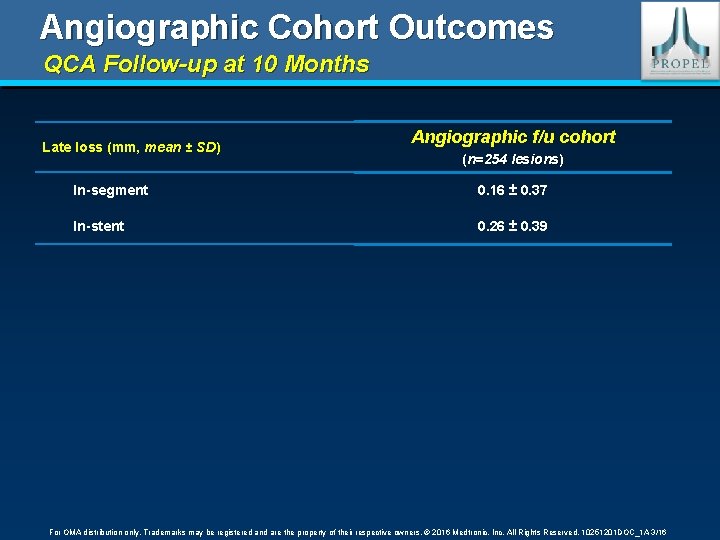
Angiographic Cohort Outcomes QCA Follow-up at 10 Months Late loss (mm, mean ± SD) SD Angiographic f/u cohort (n=254 lesions) lesions In-segment 0. 16 ± 0. 37 In-stent 0. 26 ± 0. 39 For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

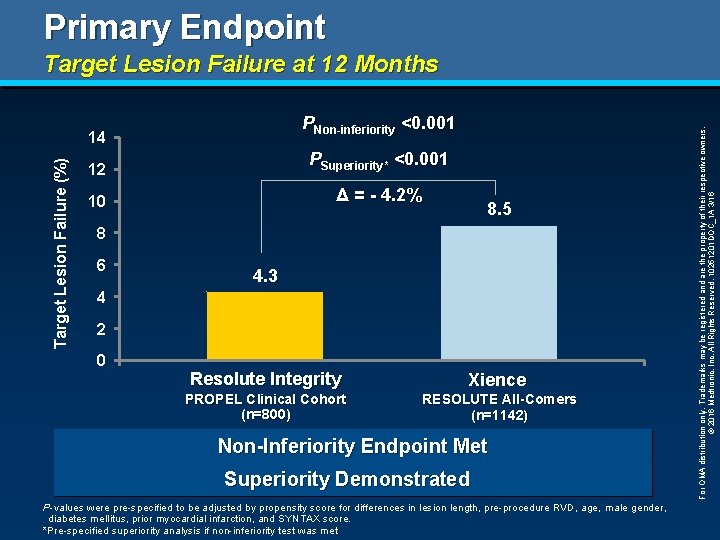
Primary Endpoint PNon-inferiority <0. 001 Target Lesion Failure (%) 14 12 PSuperiority* <0. 001 10 Δ = - 4. 2% 8. 5 8 6 4. 3 4 2 0 Resolute Integrity Xience PROPEL Clinical Cohort (n=800) RESOLUTE All-Comers (n=1142) Non-Inferiority Endpoint Met Superiority Demonstrated P-values were pre-specified to be adjusted by propensity score for differences in lesion length, pre-procedure RVD, age, male gender, diabetes mellitus, prior myocardial infarction, and SYNTAX score. *Pre-specified superiority analysis if non-inferiority test was met For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16 Target Lesion Failure at 12 Months

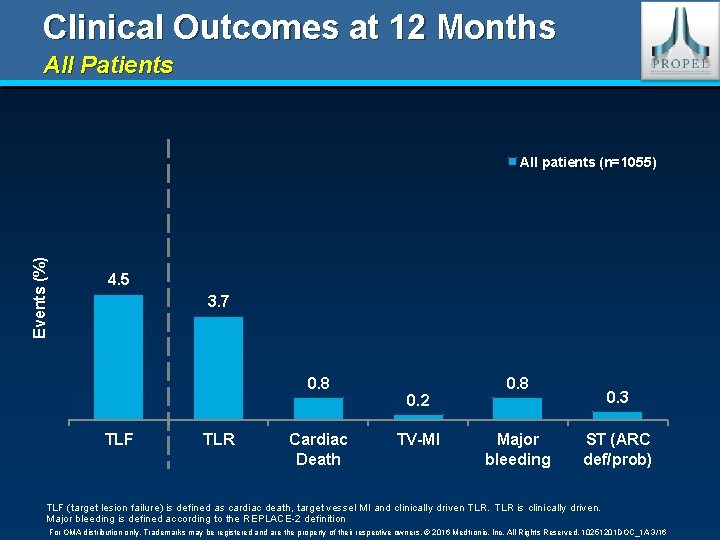
Clinical Outcomes at 12 Months All Patients Events (%) All patients (n=1055) 4. 5 3. 7 0. 8 TLF TLR Cardiac Death 0. 2 TV-MI 0. 8 Major bleeding 0. 3 ST (ARC def/prob) TLF (target lesion failure) is defined as cardiac death, target vessel MI and clinically driven TLR is clinically driven. Major bleeding is defined according to the REPLACE-2 definition For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

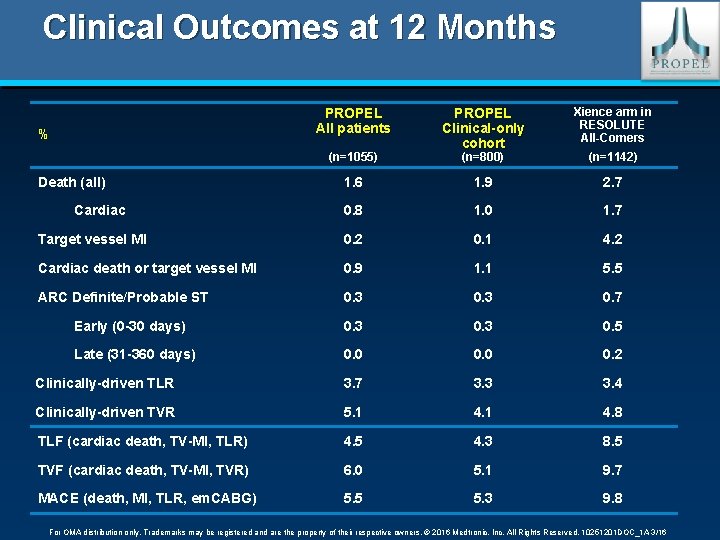
Clinical Outcomes at 12 Months PROPEL All patients PROPEL Clinical-only cohort Xience arm in RESOLUTE All-Comers (n=800) (n=1142) 1. 6 1. 9 2. 7 0. 8 1. 0 1. 7 Target vessel MI 0. 2 0. 1 4. 2 Cardiac death or target vessel MI 0. 9 1. 1 5. 5 ARC Definite/Probable ST 0. 3 0. 7 Early (0 -30 days) 0. 3 0. 5 Late (31 -360 days) 0. 0 0. 2 Clinically-driven TLR 3. 7 3. 3 3. 4 Clinically-driven TVR 5. 1 4. 8 TLF (cardiac death, TV-MI, TLR) 4. 5 4. 3 8. 5 TVF (cardiac death, TV-MI, TVR) 6. 0 5. 1 9. 7 MACE (death, MI, TLR, em. CABG) 5. 5 5. 3 9. 8 % (n=1055) Death (all) Cardiac For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

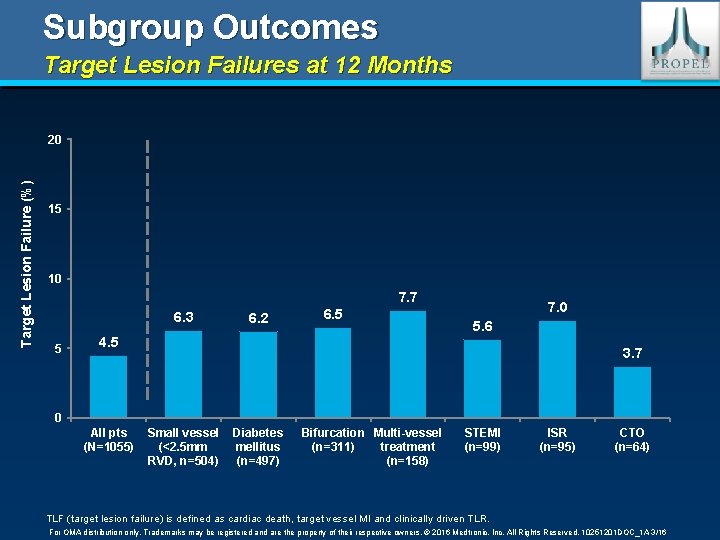
Subgroup Outcomes Target Lesion Failures at 12 Months Target Lesion Failure (%) 20 15 10 7. 7 6. 3 5 6. 2 6. 5 4. 5 7. 0 5. 6 3. 7 0 All pts (N=1055) Small vessel (<2. 5 mm RVD, n=504) Diabetes mellitus (n=497) Bifurcation Multi-vessel (n=311) treatment (n=158) STEMI (n=99) ISR (n=95) CTO (n=64) TLF (target lesion failure) is defined as cardiac death, target vessel MI and clinically driven TLR. For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

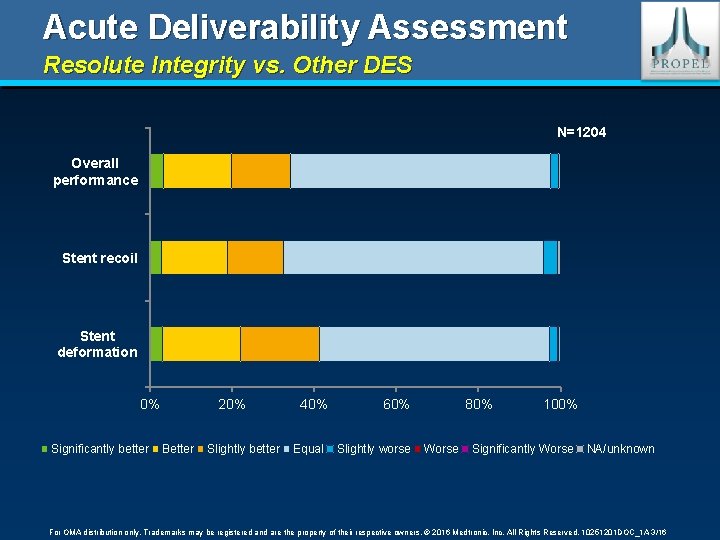
Acute Deliverability Assessment Resolute Integrity vs. Other DES N=1204 Overall performance Stent recoil Stent deformation 0% Significantly better 20% Better Slightly better 40% Equal 60% Slightly worse 80% Worse 100% Significantly Worse NA/unknown For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16

Conclusions § This is the first study to demonstrate superiority of a new generation DES (Resolute Integrity) to the benchmark Xience DES in a historical comparison (TLF 4. 3% vs 8. 5%, P<0. 001). § Procedural outcomes were excellent with lesion and procedure success rates at 100% and 99%, respectively. § At 12 months, the incidence of clinical events was low in all patients and also across various subgroups. § The Resolute Integrity stent with the Continuous Sinusoidal Technology had improved deliverability and excellent clinical outcomes. For OMA distribution only. Trademarks may be registered and are the property of their respective owners. © 2016 Medtronic, Inc. All Rights Reserved. 10251201 DOC_1 A 3/16
 Resolute integrity stent
Resolute integrity stent Stahl
Stahl You are good and your mercy is forever
You are good and your mercy is forever Ultraview lasik
Ultraview lasik Iliac stent complications
Iliac stent complications Tryton stent
Tryton stent Stent i urinleder
Stent i urinleder Fanelli stent
Fanelli stent Stent auto expansible
Stent auto expansible Pk papyrus covered coronary stent system
Pk papyrus covered coronary stent system Recidief pancreatitis
Recidief pancreatitis Stent placement
Stent placement Stent graft
Stent graft Stent placement
Stent placement Bioresorbable vascular scaffold
Bioresorbable vascular scaffold Bioresorbable stent
Bioresorbable stent Papyrus covered stent
Papyrus covered stent Magnesium stent
Magnesium stent