Clinical and Angiographic End Point Among the Intended





- Slides: 35

Clinical and Angiographic End Point Among the Intended Population of Patients with Side Branch >2. 25 mm: Results from the Randomized TRYTON Bifurcation Trial in de novo True Bifurcation Coronary Lesions Philippe Généreux, MD for the Tryton Bifurcation Trial Investigators Columbia University Medical Center Cardiovascular Research Foundation New York City Tuesday, February 25 th, 2013

Philippe Genereux, MD I/we have no real or apparent conflicts of interest to report.

Disclosure Statement of Financial Interest CRT 2014 Philippe Généreux, MD Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Research Support • None • Abbott Vascular, Cardiovascular • Consulting Fees/Honoraria • Major Stock Shareholder/Equity System Inc, • None

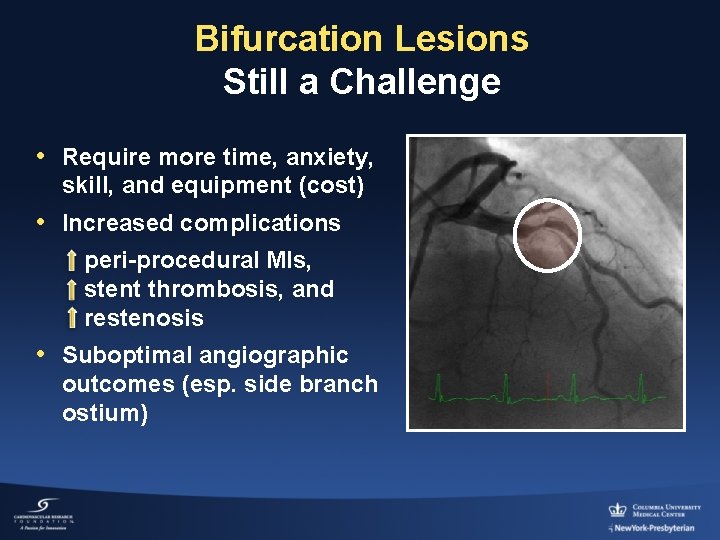
Bifurcation Lesions Still a Challenge • Require more time, anxiety, skill, and equipment (cost) • Increased complications peri-procedural MIs, stent thrombosis, and restenosis • Suboptimal angiographic outcomes (esp. side branch ostium)

Randomized Bifurcation Stent Studies (NORDIC, BBC ONE, CACTUS)

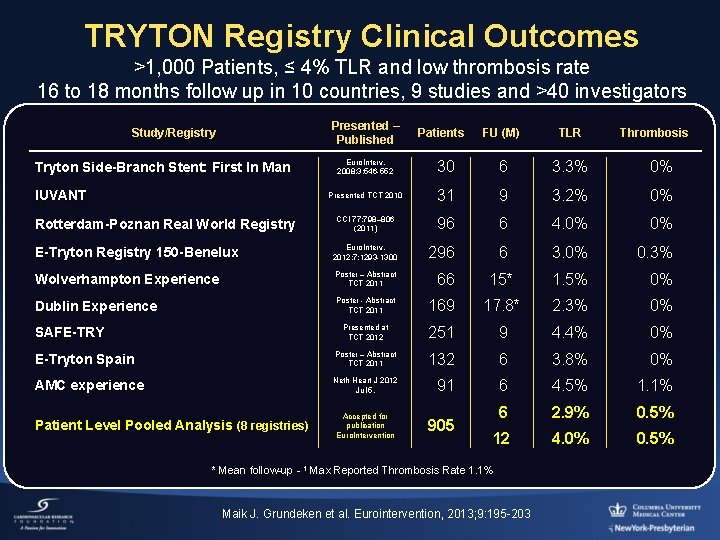
TRYTON Registry Clinical Outcomes >1, 000 Patients, ≤ 4% TLR and low thrombosis rate 16 to 18 months follow up in 10 countries, 9 studies and >40 investigators Presented – Published Patients FU (M) TLR Thrombosis Euro. Interv. 2008; 3: 546 -552 30 6 3. 3% 0% Presented TCT 2010 31 9 3. 2% 0% CCI 77: 798– 806 (2011) 96 6 4. 0% 0% E-Tryton Registry 150 -Benelux Euro. Interv. 2012; 7: 1293 -1300 296 6 3. 0% 0. 3% Wolverhampton Experience Poster – Abstract TCT 2011 66 15* 1. 5% 0% Dublin Experience Poster - Abstract TCT 2011 169 17. 8* 2. 3% 0% Presented at TCT 2012 251 9 4. 4% 0% E-Tryton Spain Poster – Abstract TCT 2011 132 6 3. 8% 0% AMC experience Neth Heart J 2012 Jul 5. 91 6 4. 5% 1. 1% Accepted for publication Euro. Intervention 905 6 2. 9% 0. 5% 12 4. 0% 0. 5% Study/Registry Tryton Side-Branch Stent: First In Man IUVANT Rotterdam-Poznan Real World Registry SAFE-TRY Patient Level Pooled Analysis (8 registries) * Mean follow-up - 1 Max Reported Thrombosis Rate 1. 1% Maik J. Grundeken et al. Eurointervention, 2013; 9: 195 -203

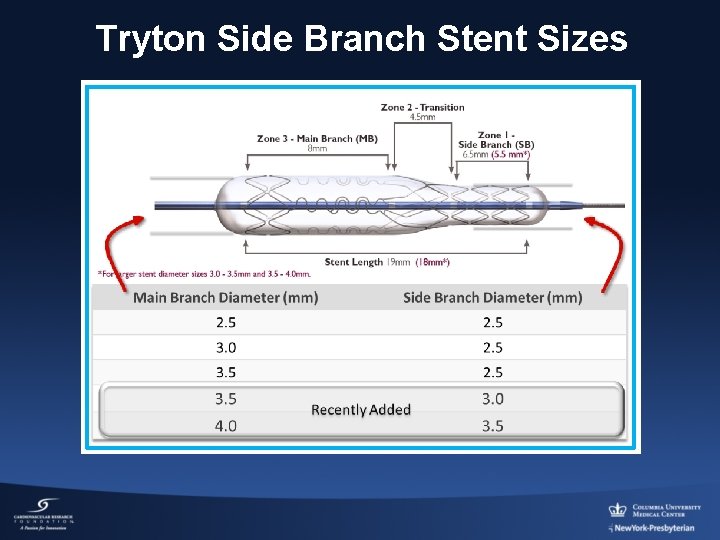
Tryton Side Branch Stent 8 mm Main Branch Zone 4. 5 mm 5. 5 -6. 5 mm Transition Zone Side Branch Zone Tryton is a Cobalt alloy bare metal stent

Tryton Side Branch Stent Sizes

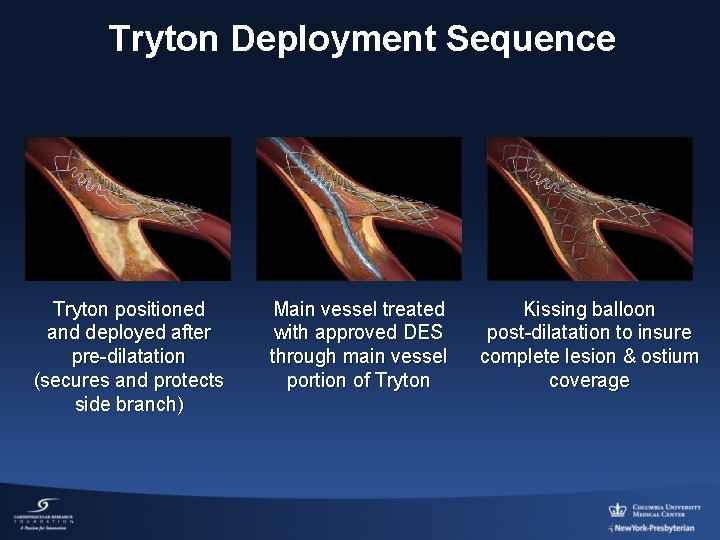
Tryton Deployment Sequence Tryton positioned and deployed after pre-dilatation (secures and protects side branch) Main vessel treated with approved DES through main vessel portion of Tryton Kissing balloon post-dilatation to insure complete lesion & ostium coverage

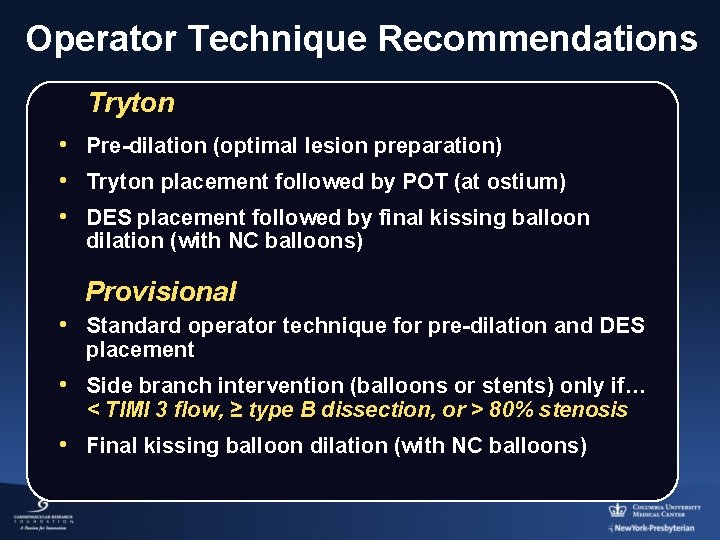
Operator Technique Recommendations Tryton • Pre-dilation (optimal lesion preparation) • Tryton placement followed by POT (at ostium) • DES placement followed by final kissing balloon dilation (with NC balloons) Provisional • Standard operator technique for pre-dilation and DES placement • Side branch intervention (balloons or stents) only if… < TIMI 3 flow, ≥ type B dissection, or > 80% stenosis • Final kissing balloon dilation (with NC balloons)

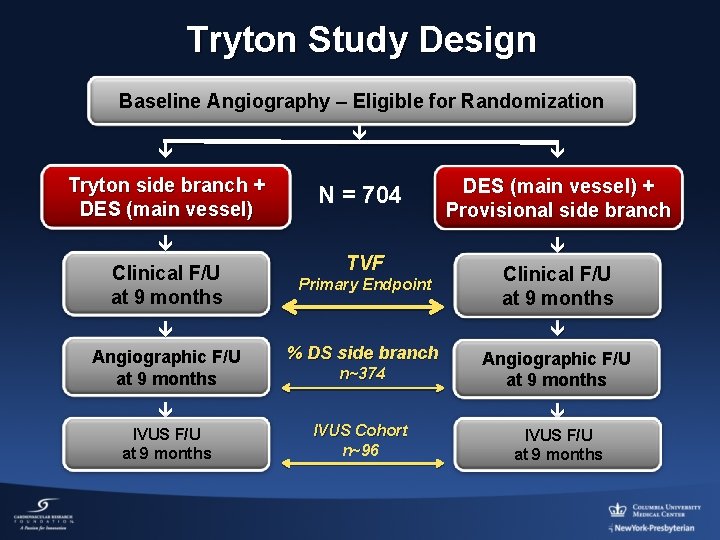
Tryton Study Design Baseline Angiography – Eligible for Randomization Tryton side branch + DES (main vessel) Clinical F/U at 9 months Angiographic F/U at 9 months IVUS F/U at 9 months N = 704 TVF Primary Endpoint DES (main vessel) + Provisional side branch Clinical F/U at 9 months % DS side branch n~374 IVUS Cohort n~96 Angiographic F/U at 9 months IVUS F/U at 9 months

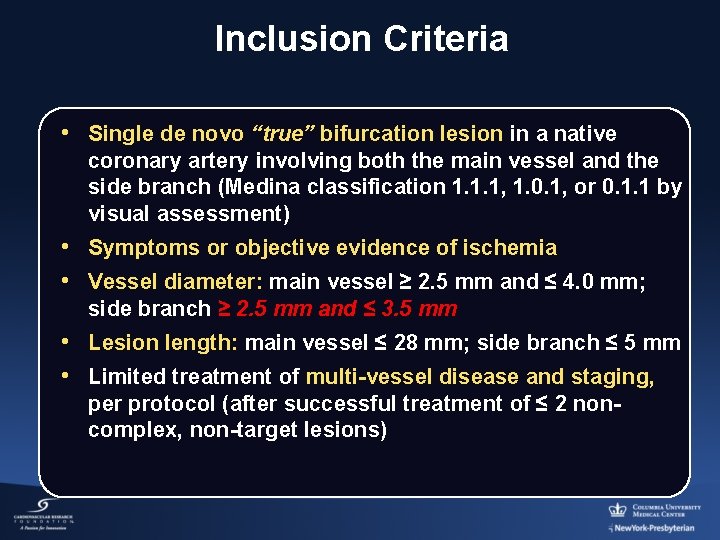
Inclusion Criteria • Single de novo “true” bifurcation lesion in a native coronary artery involving both the main vessel and the side branch (Medina classification 1. 1. 1, 1. 0. 1, or 0. 1. 1 by visual assessment) • Symptoms or objective evidence of ischemia • Vessel diameter: main vessel ≥ 2. 5 mm and ≤ 4. 0 mm; side branch ≥ 2. 5 mm and ≤ 3. 5 mm • Lesion length: main vessel ≤ 28 mm; side branch ≤ 5 mm • Limited treatment of multi-vessel disease and staging, per protocol (after successful treatment of ≤ 2 noncomplex, non-target lesions)

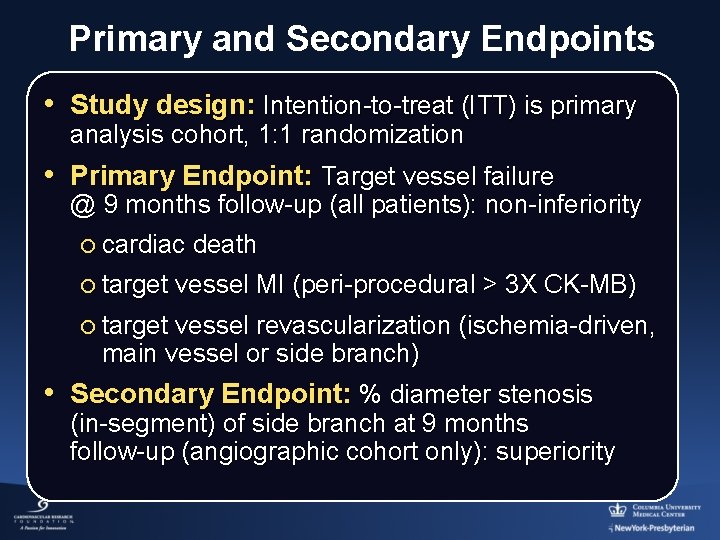
Primary and Secondary Endpoints • Study design: Intention-to-treat (ITT) is primary analysis cohort, 1: 1 randomization • Primary Endpoint: Target vessel failure @ 9 months follow-up (all patients): non-inferiority ¡ cardiac death ¡ target vessel MI (peri-procedural > 3 X CK-MB) ¡ target vessel revascularization (ischemia-driven, main vessel or side branch) • Secondary Endpoint: % diameter stenosis (in-segment) of side branch at 9 months follow-up (angiographic cohort only): superiority

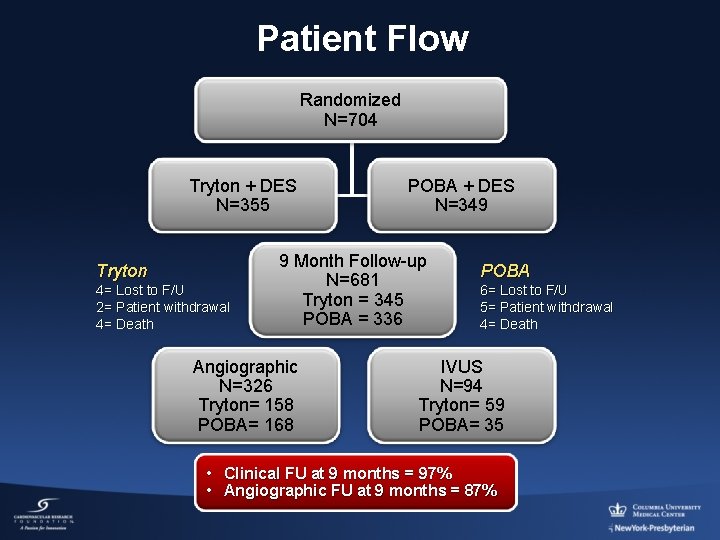
Patient Flow Randomized N=704 Tryton + DES N=355 Tryton 4= Lost to F/U 2= Patient withdrawal 4= Death POBA + DES N=349 9 Month Follow-up N=681 Tryton = 345 POBA = 336 Angiographic N=326 Tryton= 158 POBA= 168 POBA 6= Lost to F/U 5= Patient withdrawal 4= Death IVUS N=94 Tryton= 59 POBA= 35 • Clinical FU at 9 months = 97% • Angiographic FU at 9 months = 87%

Tryton Bifurcation Study Main Study Results Leon, MB, TCT 2013

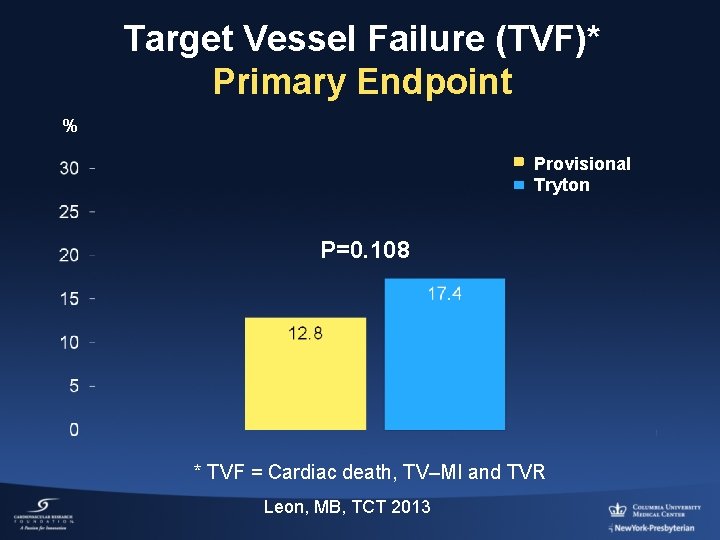
Target Vessel Failure (TVF)* Primary Endpoint % Provisional Tryton P=0. 108 * TVF = Cardiac death, TV–MI and TVR Leon, MB, TCT 2013

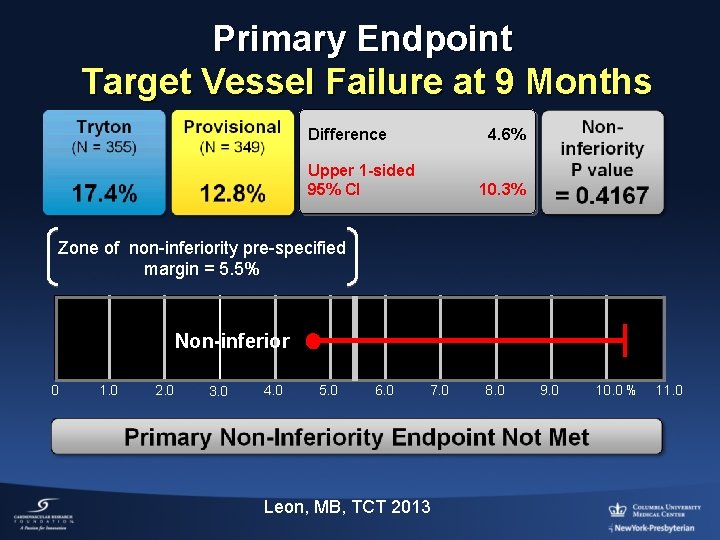
Primary Endpoint Target Vessel Failure at 9 Months Difference 4. 6% Upper 1 -sided 95% CI 10. 3% Zone of non-inferiority pre-specified margin = 5. 5% Non-inferior 0 1. 0 2. 0 3. 0 4. 0 5. 0 6. 0 7. 0 Leon, MB, TCT 2013 8. 0 9. 0 10. 0 % 11. 0

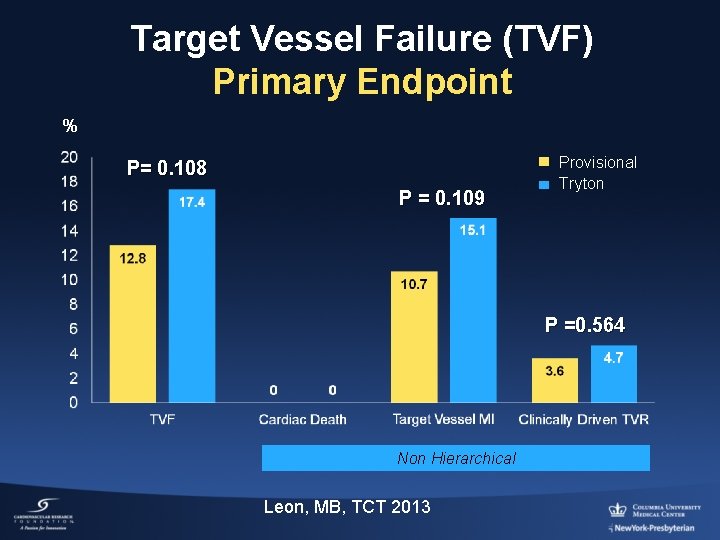
Target Vessel Failure (TVF) Primary Endpoint % P= 0. 108 P = 0. 109 Provisional Tryton P =0. 564 Non Hierarchical Leon, MB, TCT 2013

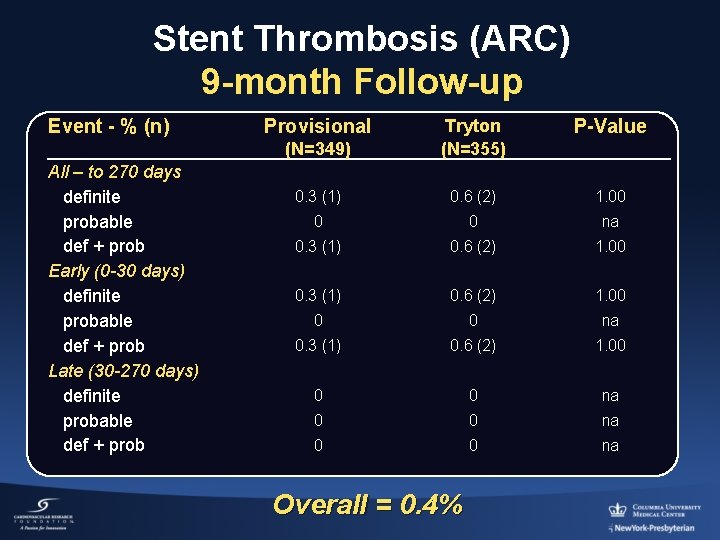
Stent Thrombosis (ARC) 9 -month Follow-up Event - % (n) All – to 270 days definite probable def + prob Early (0 -30 days) definite probable def + prob Late (30 -270 days) definite probable def + prob Provisional P-Value (N=349) Tryton (N=355) 0. 3 (1) 0. 6 (2) 1. 00 0 0 na Overall = 0. 4%

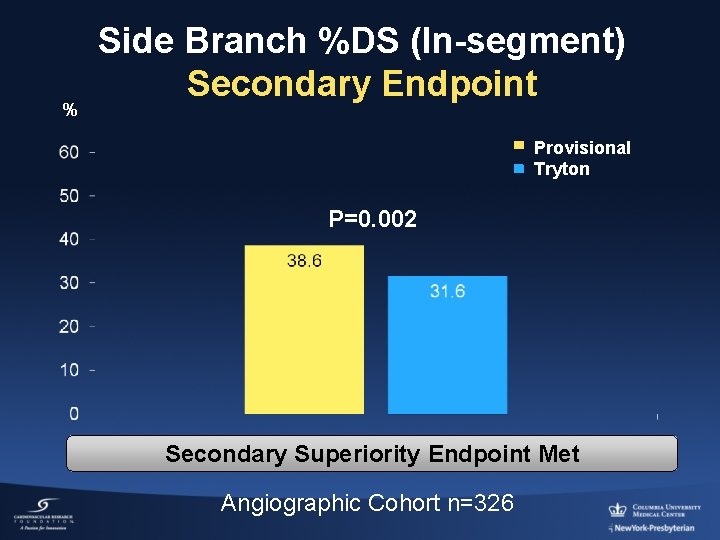
% Side Branch %DS (In-segment) Secondary Endpoint Provisional Tryton P=0. 002 Secondary Superiority Endpoint Met Angiographic Cohort n=326

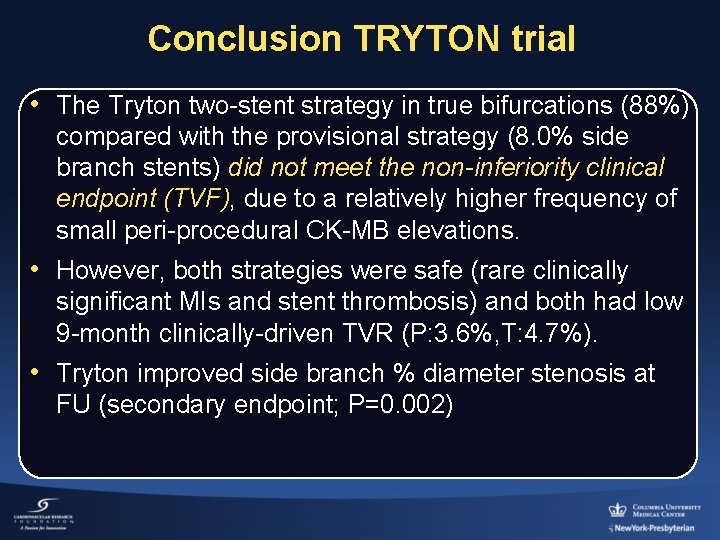
Conclusion TRYTON trial • The Tryton two-stent strategy in true bifurcations (88%) compared with the provisional strategy (8. 0% side branch stents) did not meet the non-inferiority clinical endpoint (TVF), due to a relatively higher frequency of small peri-procedural CK-MB elevations. • However, both strategies were safe (rare clinically significant MIs and stent thrombosis) and both had low 9 -month clinically-driven TVR (P: 3. 6%, T: 4. 7%). • Tryton improved side branch % diameter stenosis at FU (secondary endpoint; P=0. 002)

Tryton Bifurcation Study Side Branch Post-hoc Subset ≥ 2. 25 mm: Intended Population Analyses

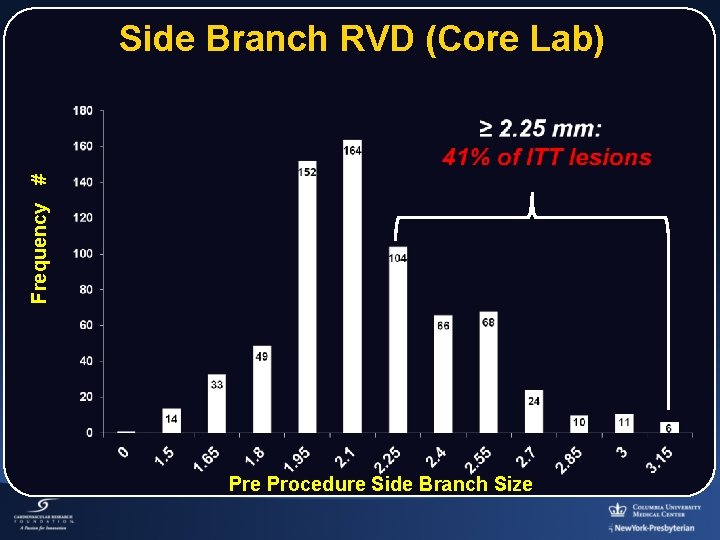
Frequency # Side Branch RVD (Core Lab) Pre Procedure Side Branch Size

Study Population: SB RVD Smaller than Intended WHY? • Recruitment Enthusiasm Drove inclusion of non-qualified lesions • Tryton SB SDS Size Limitations ≥ 2. 5 mm SB Tryton: SB stent delivery system ≥ 2. 5 mm Provisional: PTCA Balloons ≥ 2. 0 mm • Lesions Involving Small SB Smaller Myocardium at Risk Investigator Equipoise More Likely

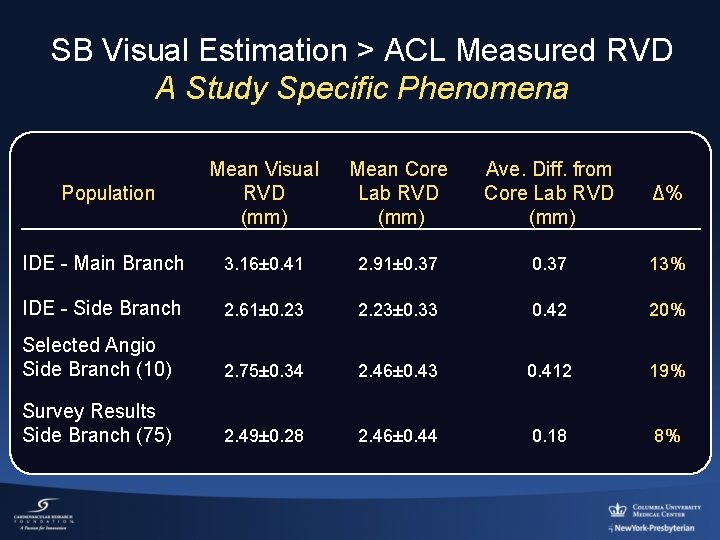
SB Visual Estimation > ACL Measured RVD A Study Specific Phenomena Population Mean Visual Mean Core RVD Lab RVD (mm) Ave. Diff. from Core Lab RVD (mm) Δ% IDE - Main Branch 3. 16± 0. 41 2. 91± 0. 37 13% IDE - Side Branch 2. 61± 0. 23 2. 23± 0. 33 0. 42 20% Selected Angio Side Branch (10) 2. 75± 0. 34 2. 46± 0. 43 0. 412 19% Survey Results Side Branch (75) 2. 49± 0. 28 2. 46± 0. 44 0. 18 8%

Tryton Bifurcation Study Side Branch ≥ 2. 25 mm: Results

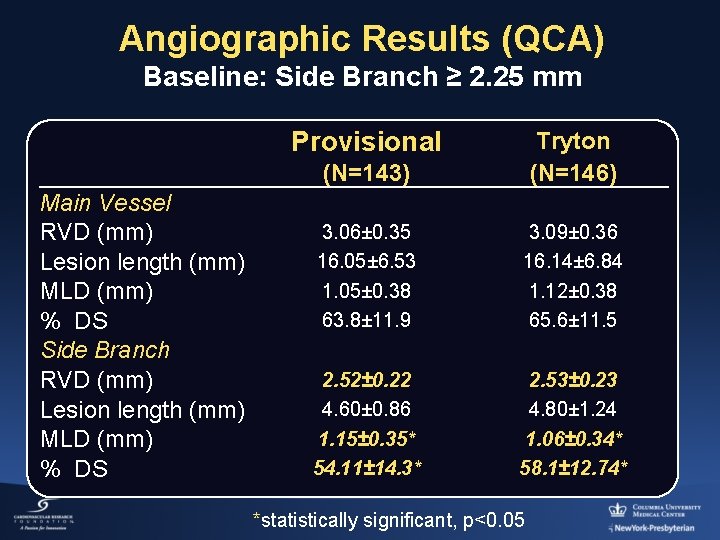
Angiographic Results (QCA) Baseline: Side Branch ≥ 2. 25 mm Provisional Main Vessel RVD (mm) Lesion length (mm) MLD (mm) % DS Side Branch RVD (mm) Lesion length (mm) MLD (mm) % DS (N=143) Tryton (N=146) 3. 06± 0. 35 16. 05± 6. 53 1. 05± 0. 38 63. 8± 11. 9 3. 09± 0. 36 16. 14± 6. 84 1. 12± 0. 38 65. 6± 11. 5 2. 52± 0. 22 4. 60± 0. 86 1. 15± 0. 35* 54. 11± 14. 3* 2. 53± 0. 23 4. 80± 1. 24 1. 06± 0. 34* 58. 1± 12. 74* *statistically significant, p<0. 05

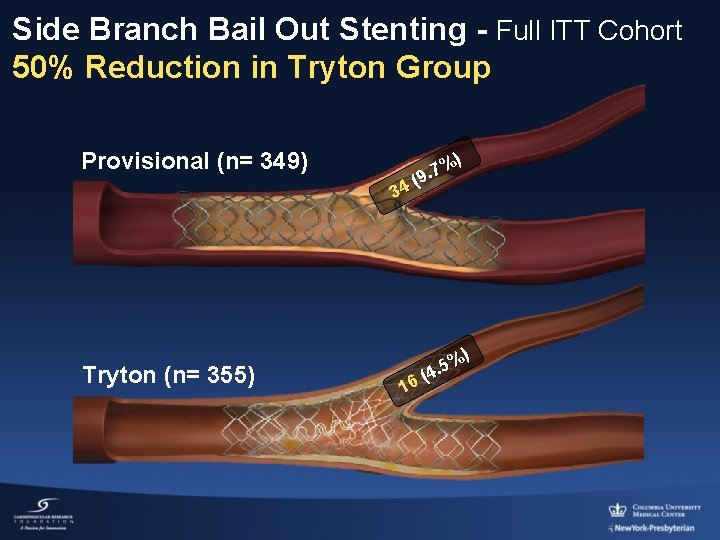
Side Branch Bail Out Stenting - Full ITT Cohort 50% Reduction in Tryton Group Provisional (n= 349) 34 Tryton (n= 355) %) 7. (9 16 %) 5. (4

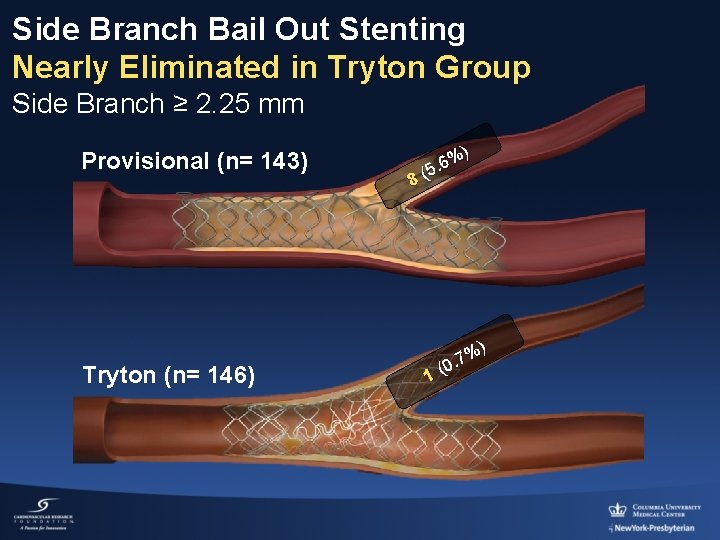
Side Branch Bail Out Stenting Nearly Eliminated in Tryton Group Side Branch ≥ 2. 25 mm Provisional (n= 143) Tryton (n= 146) ) % 6 5. 8( 1 %) 7. (0

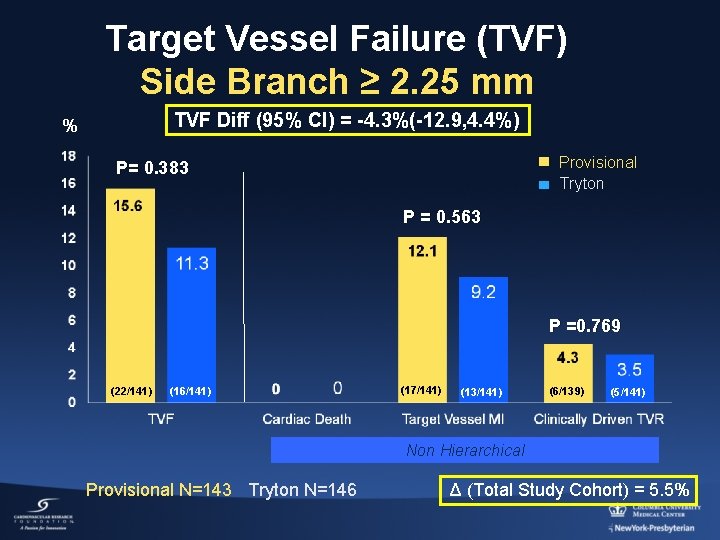
Target Vessel Failure (TVF) Side Branch ≥ 2. 25 mm TVF Diff (95% CI) = -4. 3%(-12. 9, 4. 4%) % Provisional Tryton P= 0. 383 P = 0. 563 P =0. 769 (22/141) (16/141) (17/141) (13/141) (6/139) (5/141) Non Hierarchical Provisional N=143 Tryton N=146 Δ (Total Study Cohort) = 5. 5%

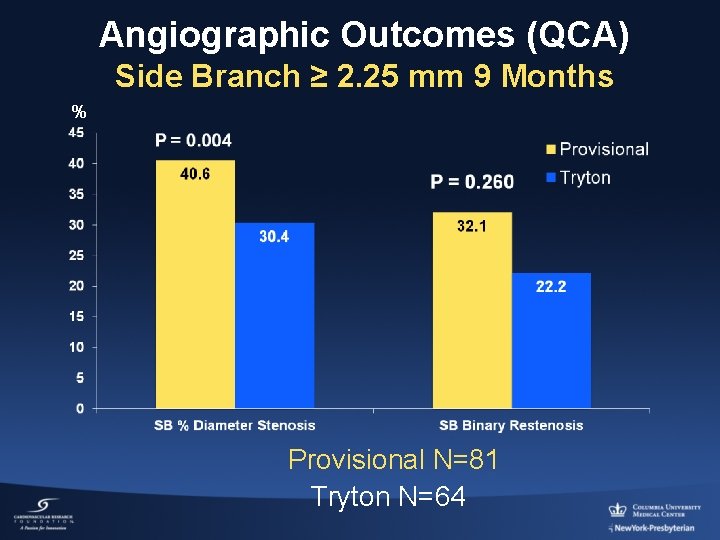
Angiographic Outcomes (QCA) Side Branch ≥ 2. 25 mm 9 Months % Provisional N=81 Tryton N=64

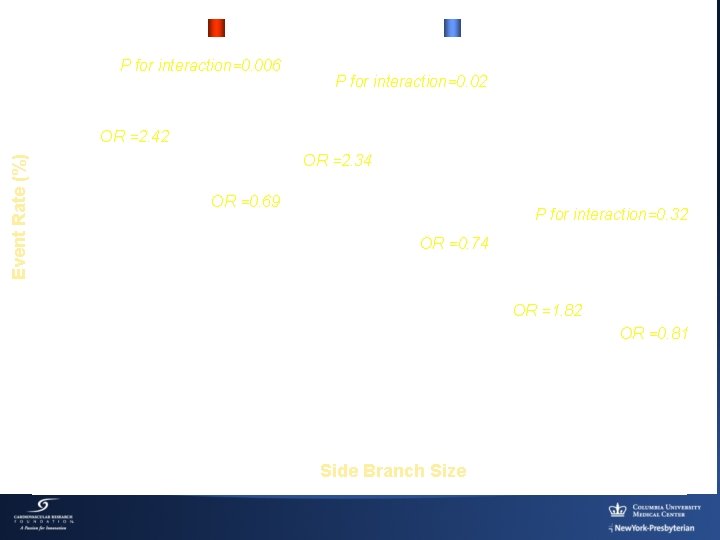
Provisional P for interaction=0. 006 Event Rate (%) TVF OR =2. 42 [1. 37, 4. 28] TRYTON Stent P for interaction=0. 02 Target Vessel MI OR =2. 34 [1. 29, 4. 25] OR =0. 69 [0. 35, 1. 38] P for interaction=0. 32 OR =0. 74 [0. 35, 1. 59] Clinically Driven TVR OR =1. 82 [0. 66, 5. 03] 20/195 44/203 22/141 16/141 18/195 39/203 17/141 13/141 Side Branch Size 6/195 11/201 OR =0. 81 [0. 24, 2. 73] 6/139 5/141

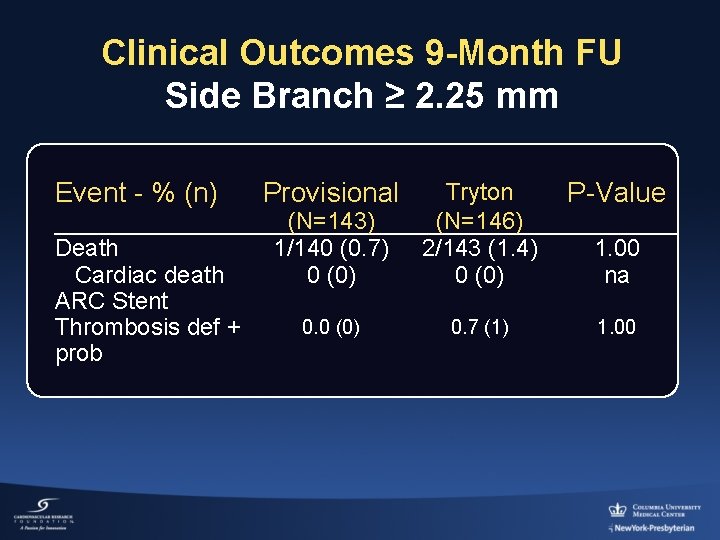
Clinical Outcomes 9 -Month FU Side Branch ≥ 2. 25 mm Event - % (n) Death Cardiac death ARC Stent Thrombosis def + prob Provisional (N=143) 1/140 (0. 7) 0 (0) Tryton (N=146) 2/143 (1. 4) 0 (0) P-Value 0. 0 (0) 0. 7 (1) 1. 00 na

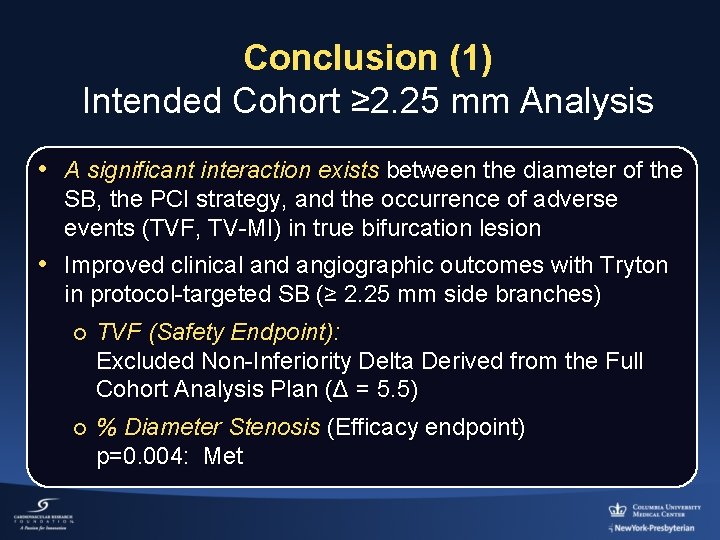
Conclusion (1) Intended Cohort ≥ 2. 25 mm Analysis • A significant interaction exists between the diameter of the SB, the PCI strategy, and the occurrence of adverse events (TVF, TV-MI) in true bifurcation lesion • Improved clinical and angiographic outcomes with Tryton in protocol-targeted SB (≥ 2. 25 mm side branches) ¡ TVF (Safety Endpoint): Excluded Non-Inferiority Delta Derived from the Full Cohort Analysis Plan (Δ = 5. 5) ¡ % Diameter Stenosis (Efficacy endpoint) p=0. 004: Met

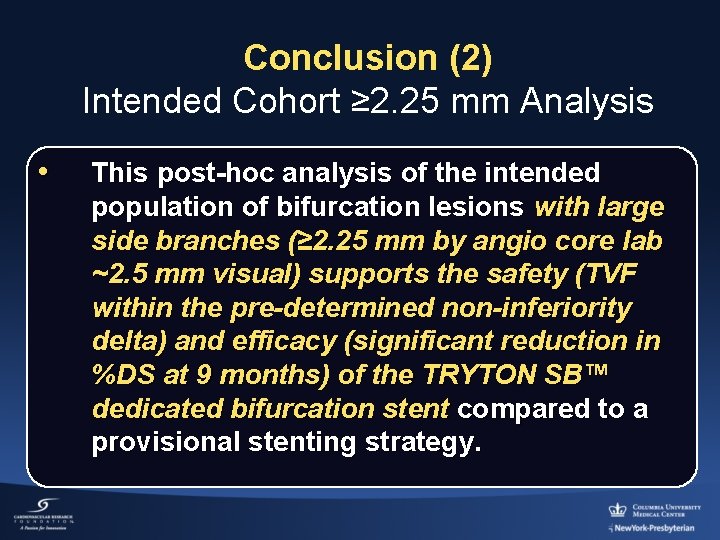
Conclusion (2) Intended Cohort ≥ 2. 25 mm Analysis • This post-hoc analysis of the intended population of bifurcation lesions with large side branches (≥ 2. 25 mm by angio core lab ~2. 5 mm visual) supports the safety (TVF within the pre-determined non-inferiority delta) and efficacy (significant reduction in %DS at 9 months) of the TRYTON SB™ dedicated bifurcation stent compared to a provisional stenting strategy.
 Angiographic equipment
Angiographic equipment Angiography equipment list
Angiography equipment list End point and equivalence point
End point and equivalence point Blocked random assignment
Blocked random assignment Explain compiler construction tools
Explain compiler construction tools Explain compiler construction tools
Explain compiler construction tools Alpena biorefinery
Alpena biorefinery Fuses and circuit breakers are intended primarily for the
Fuses and circuit breakers are intended primarily for the End-diastolic volume vs end-systolic volume
End-diastolic volume vs end-systolic volume End-diastolic volume vs end-systolic volume
End-diastolic volume vs end-systolic volume Feride kröpil
Feride kröpil Yichao zhou
Yichao zhou End to end argument
End to end argument End to end accounting life cycle tasks
End to end accounting life cycle tasks End to end delay
End to end delay End to end delay
End to end delay End to end
End to end Comet commonsense
Comet commonsense Multiple procurement cycles
Multiple procurement cycles Difference between endpoint and kinetic method
Difference between endpoint and kinetic method Urine osmolality calculator
Urine osmolality calculator Airline hub model
Airline hub model It has 4-5 inches finely tapered blades
It has 4-5 inches finely tapered blades Literary devices romeo and juliet
Literary devices romeo and juliet What is intended benefit
What is intended benefit It simulate or animate some features of intended system
It simulate or animate some features of intended system Intended curriculum
Intended curriculum Ibis in hci
Ibis in hci Tasmanian bdm
Tasmanian bdm What is the purpose of the text?
What is the purpose of the text? Intended outcome
Intended outcome It is the intended height of the function?
It is the intended height of the function? In her statement (lines 53-54) miss nightingale intended to
In her statement (lines 53-54) miss nightingale intended to Most inhalants are actually intended to be
Most inhalants are actually intended to be Hippo apush meaning
Hippo apush meaning Direct and indirect voluntariness example
Direct and indirect voluntariness example