Naming the Inorganic Compounds Kavita Gupta Classification of

- Slides: 12

Naming the Inorganic Compounds Kavita Gupta

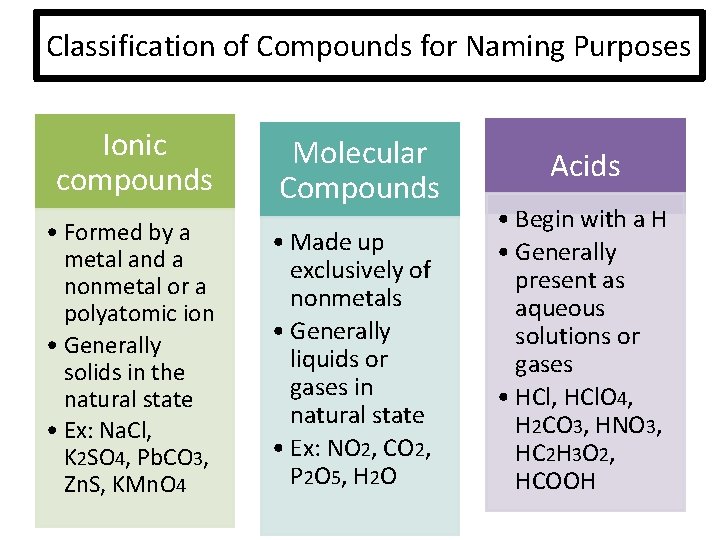
Classification of Compounds for Naming Purposes Ionic compounds • Formed by a metal and a nonmetal or a polyatomic ion • Generally solids in the natural state • Ex: Na. Cl, K 2 SO 4, Pb. CO 3, Zn. S, KMn. O 4 Molecular Compounds • Made up exclusively of nonmetals • Generally liquids or gases in natural state • Ex: NO 2, CO 2, P 2 O 5 , H 2 O Acids • Begin with a H • Generally present as aqueous solutions or gases • HCl, HCl. O 4, H 2 CO 3, HNO 3, HC 2 H 3 O 2, HCOOH

www. fransonchiropractic. com/blog/2010

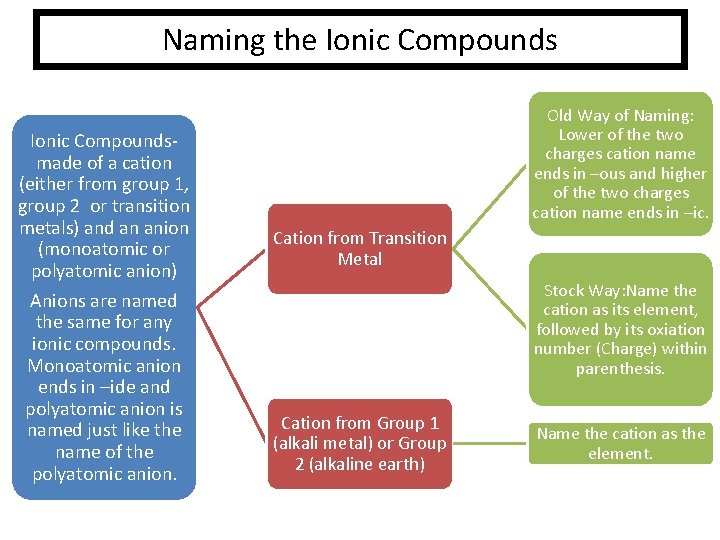
Naming the Ionic Compoundsmade of a cation (either from group 1, group 2 or transition metals) and an anion (monoatomic or polyatomic anion) Anions are named the same for any ionic compounds. Monoatomic anion ends in –ide and polyatomic anion is named just like the name of the polyatomic anion. Old Way of Naming: Lower of the two charges cation name ends in –ous and higher of the two charges cation name ends in –ic. Cation from Transition Metal Stock Way: Name the cation as its element, followed by its oxiation number (Charge) within parenthesis. Cation from Group 1 (alkali metal) or Group 2 (alkaline earth) Name the cation as the element.

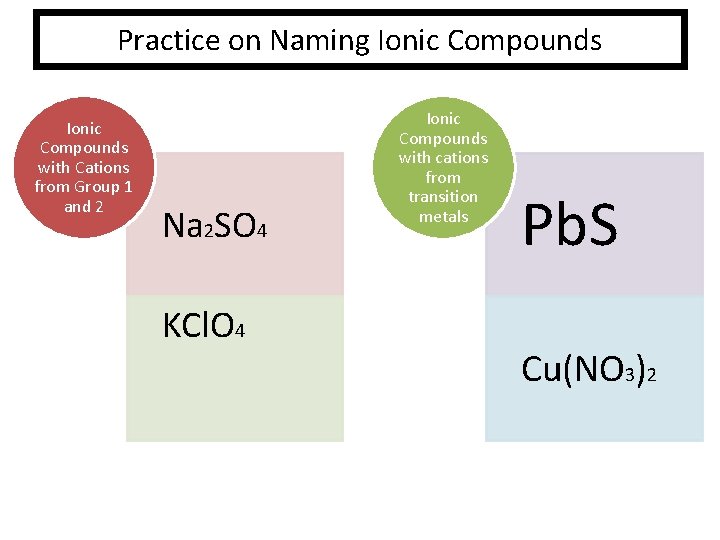
Practice on Naming Ionic Compounds with Cations from Group 1 and 2 Na 2 SO 4 KCl. O 4 Ionic Compounds with cations from transition metals Pb. S Cu(NO 3)2

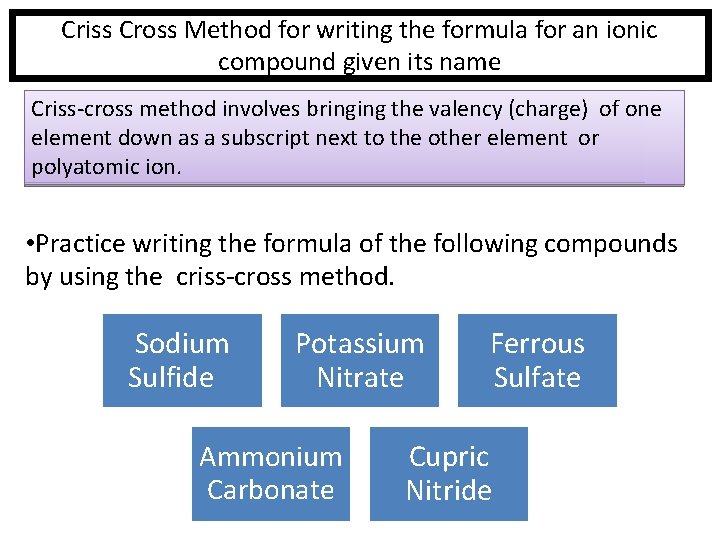
Criss Cross Method for writing the formula for an ionic compound given its name Criss-cross method involves bringing the valency (charge) of one element down as a subscript next to the other element or polyatomic ion. • Practice writing the formula of the following compounds by using the criss-cross method. Sodium Sulfide Potassium Nitrate Ammonium Carbonate Ferrous Sulfate Cupric Nitride

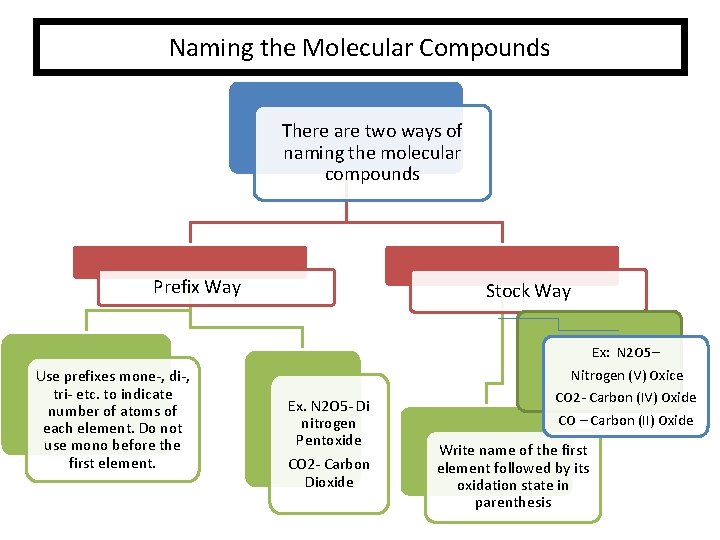
Naming the Molecular Compounds There are two ways of naming the molecular compounds Prefix Way Use prefixes mone-, di-, tri- etc. to indicate number of atoms of each element. Do not use mono before the first element. Stock Way Ex. N 2 O 5 - Di nitrogen Pentoxide CO 2 - Carbon Dioxide Ex: N 2 O 5– Nitrogen (V) Oxice CO 2 - Carbon (IV) Oxide CO – Carbon (II) Oxide Write name of the first element followed by its oxidation state in parenthesis

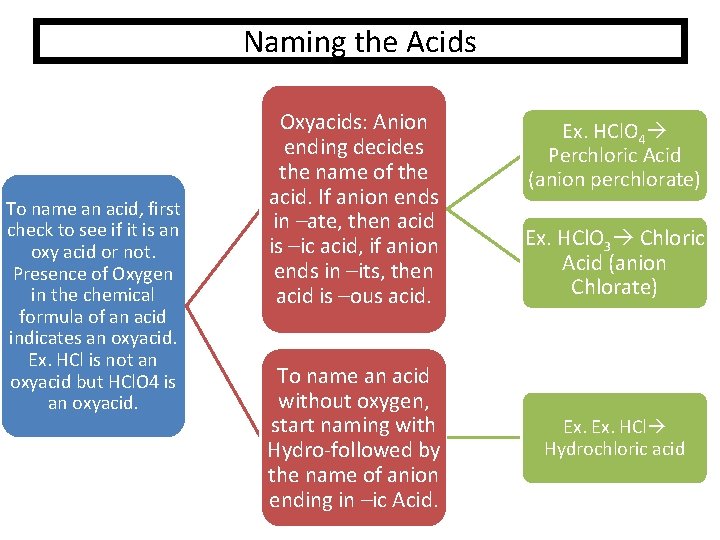
Naming the Acids To name an acid, first check to see if it is an oxy acid or not. Presence of Oxygen in the chemical formula of an acid indicates an oxyacid. Ex. HCl is not an oxyacid but HCl. O 4 is an oxyacid. Oxyacids: Anion ending decides the name of the acid. If anion ends in –ate, then acid is –ic acid, if anion ends in –its, then acid is –ous acid. To name an acid without oxygen, start naming with Hydro-followed by the name of anion ending in –ic Acid. Ex. HCl. O 4 Perchloric Acid (anion perchlorate) Ex. HCl. O 3 Chloric Acid (anion Chlorate) Ex. HCl Hydrochloric acid

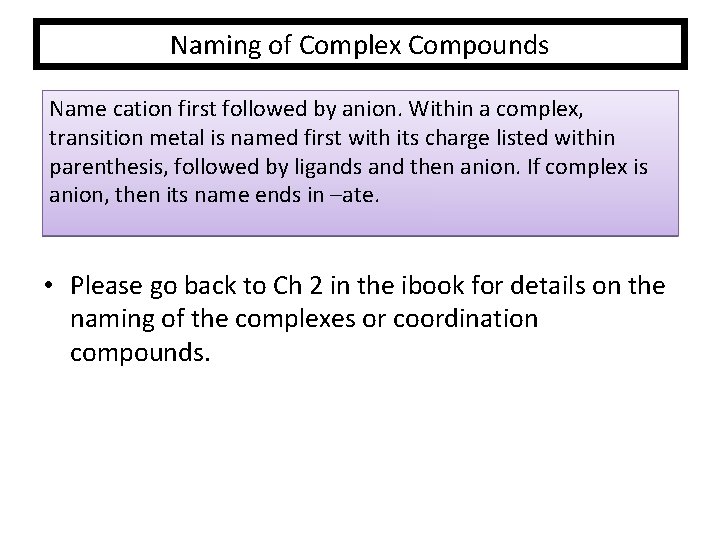
Naming of Complex Compounds Name cation first followed by anion. Within a complex, transition metal is named first with its charge listed within parenthesis, followed by ligands and then anion. If complex is anion, then its name ends in –ate. • Please go back to Ch 2 in the ibook for details on the naming of the complexes or coordination compounds.

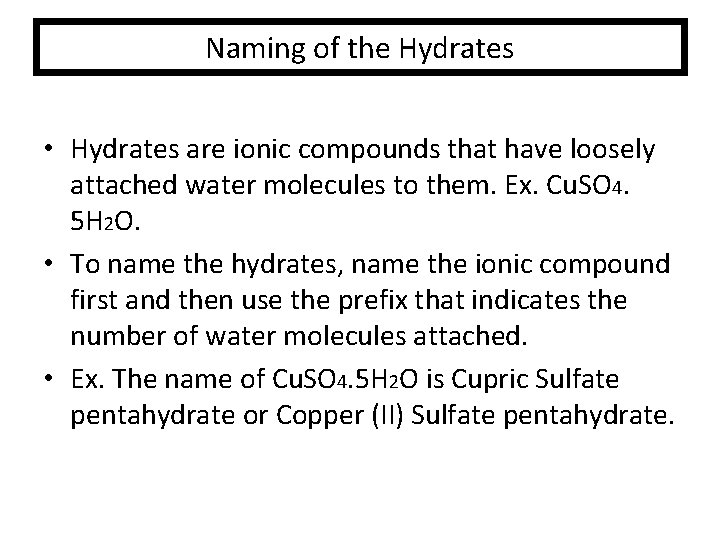
Naming of the Hydrates • Hydrates are ionic compounds that have loosely attached water molecules to them. Ex. Cu. SO 4. 5 H 2 O. • To name the hydrates, name the ionic compound first and then use the prefix that indicates the number of water molecules attached. • Ex. The name of Cu. SO 4. 5 H 2 O is Cupric Sulfate pentahydrate or Copper (II) Sulfate pentahydrate.

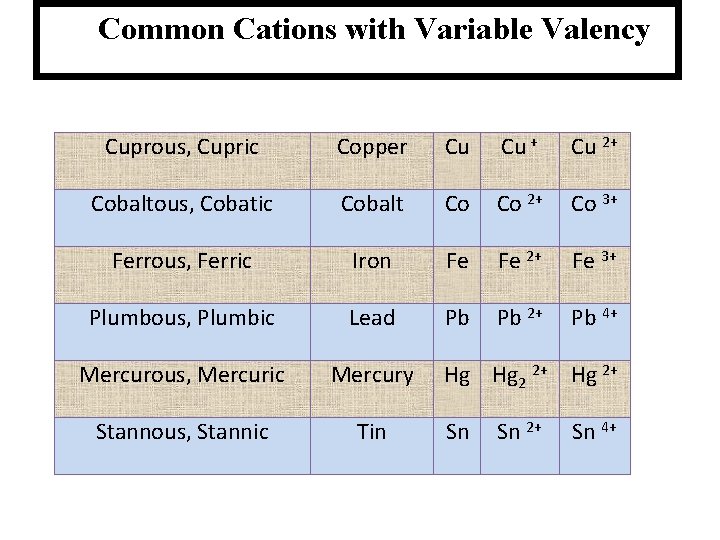
Common Cations with Variable Valency Cuprous, Cupric Copper Cu Cu + Cu 2+ Cobaltous, Cobatic Cobalt Co Co 2+ Co 3+ Ferrous, Ferric Iron Fe Fe 2+ Fe 3+ Plumbous, Plumbic Lead Pb Pb 2+ Pb 4+ Mercurous, Mercuric Mercury Stannous, Stannic Tin Hg Hg 2 2+ Hg 2+ Sn Sn 2+ Sn 4+

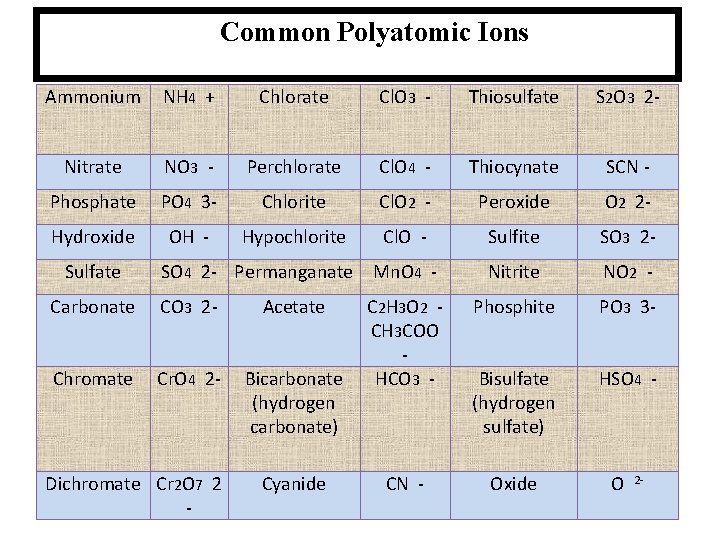
Common Polyatomic Ions Ammonium NH 4 + Chlorate Cl. O 3 - Thiosulfate S 2 O 3 2 - Nitrate NO 3 - Perchlorate Cl. O 4 - Thiocynate SCN - Phosphate PO 4 3 - Chlorite Cl. O 2 - Peroxide O 2 2 - Hydroxide OH - Hypochlorite Cl. O - Sulfite SO 3 2 - Nitrite NO 2 - Phosphite PO 3 3 - Bisulfate (hydrogen sulfate) HSO 4 - Sulfate SO 4 2 - Permanganate Mn. O 4 - Carbonate CO 3 2 - Acetate Chromate Cr. O 4 2 - Bicarbonate (hydrogen carbonate) Dichromate Cr 2 O 7 2 - Cyanide C 2 H 3 O 2 CH 3 COO HCO 3 - CN - Oxide O 2 -
 Kavita gupta md
Kavita gupta md Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Organic vs inorganic compounds
Organic vs inorganic compounds What is the difference between organic and inorganic
What is the difference between organic and inorganic Organic vs inorganic compounds
Organic vs inorganic compounds Pharmaceutical inorganic chemistry introduction
Pharmaceutical inorganic chemistry introduction Site:slidetodoc.com
Site:slidetodoc.com How to name metallic bonds
How to name metallic bonds Organic chemistry nomenclature
Organic chemistry nomenclature Ni ionic charge
Ni ionic charge Ionic name
Ionic name What is the correct name for pbr3
What is the correct name for pbr3 When to use prefixes for naming compounds
When to use prefixes for naming compounds