Most missed questions chemistry regents exam June 2016










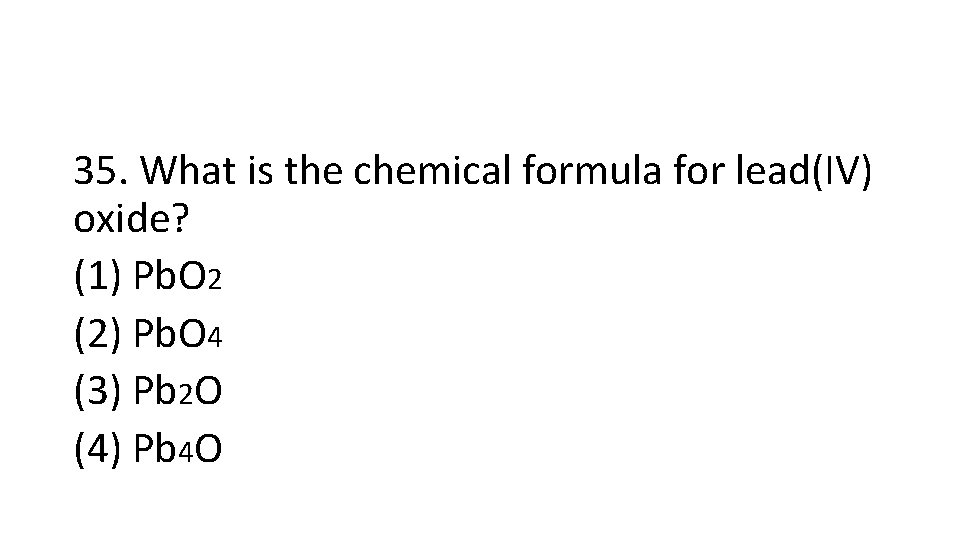




![51. Draw a Lewis electron-dot diagram for a chloride ion, Cl [1] 57. State 51. Draw a Lewis electron-dot diagram for a chloride ion, Cl [1] 57. State](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-18.jpg)
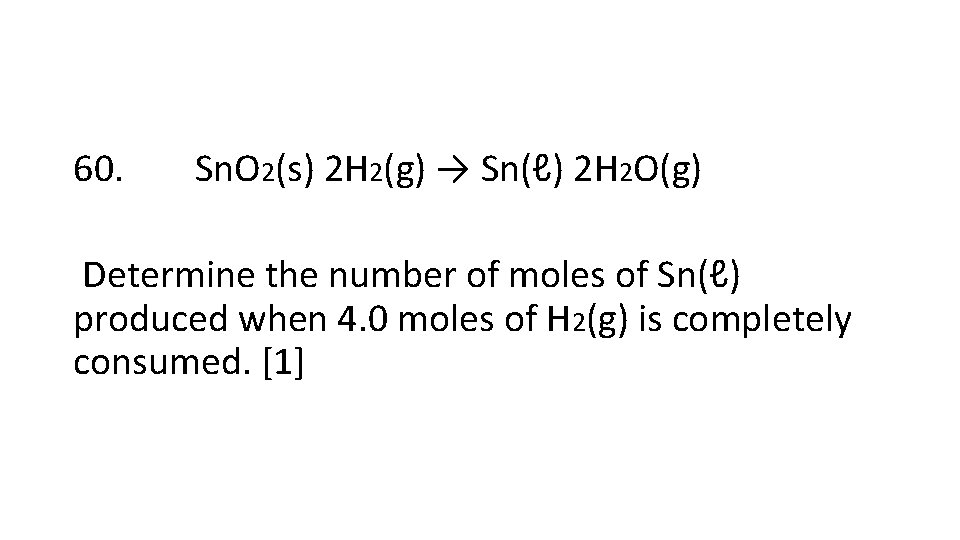

![78. State the purpose of the salt bridge in the voltaic cell. [1] 79. 78. State the purpose of the salt bridge in the voltaic cell. [1] 79.](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-21.jpg)

![84. Determine the number of neutrons in an atom of U-233. [1] 85. Identify 84. Determine the number of neutrons in an atom of U-233. [1] 85. Identify](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-23.jpg)

![Answers 51. Teacher will show on board. 57 [1] Allow 1 credit. Acceptable responses Answers 51. Teacher will show on board. 57 [1] Allow 1 credit. Acceptable responses](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-25.jpg)
![Answers 66 [1] Allow 1 credit. Acceptable responses include, but are not limited to: Answers 66 [1] Allow 1 credit. Acceptable responses include, but are not limited to:](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-26.jpg)
![Answers 78 [1] Allow 1 credit. Acceptable responses include, but are not limited to: Answers 78 [1] Allow 1 credit. Acceptable responses include, but are not limited to:](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-27.jpg)

- Slides: 28

Most missed questions chemistry regents exam June 2016

4. Which phrase describes two forms of solid carbon, diamond and graphite, at STP? (1)the same crystal structure and the same properties (2)the same crystal structure and different properties (3)different crystal structures and the same properties (4)different crystal structures and different properties

9 Which atom has the greatest attraction for the electrons in a chemical bond? (1) hydrogen (2) oxygen (3) silicon (4) sulfur

11. A 10. 0 -gram sample of nitrogen is at STP. Which property will increase when the sample is cooled to 72 K at standard pressure? (1) mass (2) volume (3) density (4) temperature

13 A 5. 0 -gram sample of Fe(s) is to be placed in 100. milliliters of HCl(aq). Which changes will result in the fastest rate of reaction? (1) increasing the surface area of Fe(s) and increasing the concentration of HCl(aq) (2) increasing the surface area of Fe(s) and decreasing the concentration of HCl(aq) (3) decreasing the surface area of Fe(s) and increasing the concentration of HCl(aq) (4) decreasing the surface area of Fe(s) and decreasing the concentration of HCl(aq)

17. At STP, which sample contains the same number of molecules as 3. 0 liters of H 2(g)? (1) 1. 5 L of NH 3(g) (2) 2. 0 L of CO 2(g) (3) 3. 0 L of CH 4(g) (4) 6. 0 L of N 2(g)

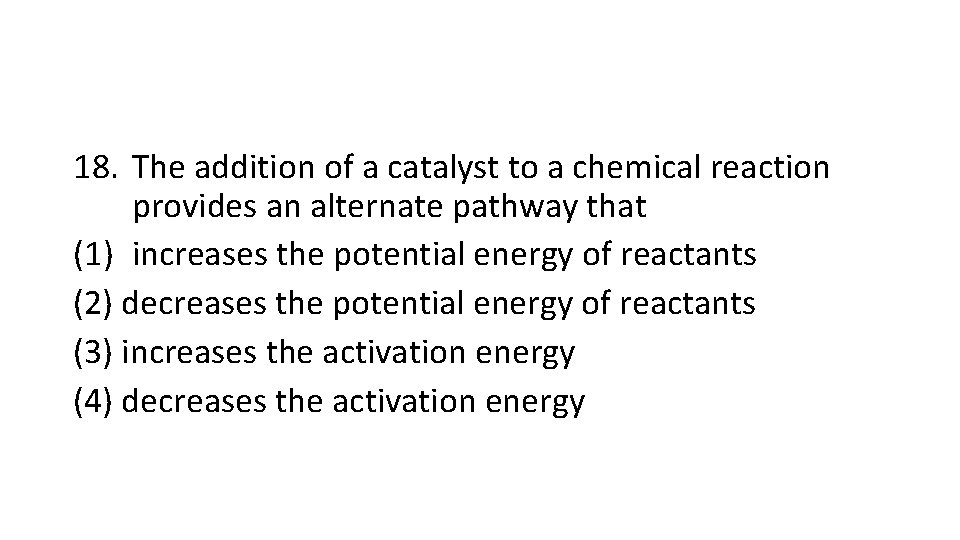
18. The addition of a catalyst to a chemical reaction provides an alternate pathway that (1) increases the potential energy of reactants (2) decreases the potential energy of reactants (3) increases the activation energy (4) decreases the activation energy

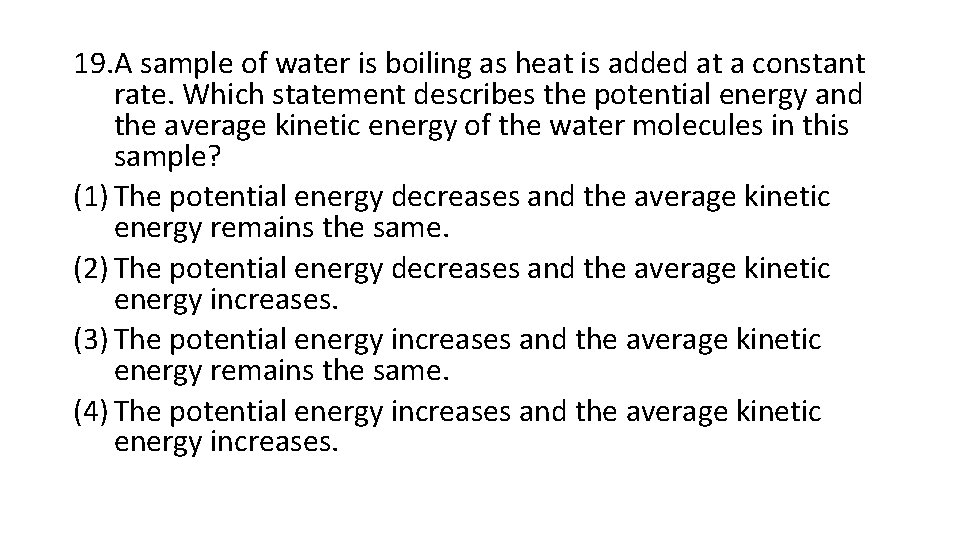
19. A sample of water is boiling as heat is added at a constant rate. Which statement describes the potential energy and the average kinetic energy of the water molecules in this sample? (1) The potential energy decreases and the average kinetic energy remains the same. (2) The potential energy decreases and the average kinetic energy increases. (3) The potential energy increases and the average kinetic energy remains the same. (4) The potential energy increases and the average kinetic energy increases.

22 What is the number of electrons shared in the multiple carbon-carbon bond in one molecule of 1 -pentyne? (1) 6 (2) 2 (3) 3 (4) 8

24 What occurs when a magnesium atom becomes a magnesium ion? (1) Electrons are gained and the oxidation number increases. (2) Electrons are gained and the oxidation number decreases. (3) Electrons are lost and the oxidation number increases. (4) Electrons are lost and the oxidation number decreases.

27. One acid-base theory defines an acid as an (1) H- acceptor (2) H- donor (3) H+ acceptor (4) H+ donor

30. Which change occurs during a nuclear fission reaction? (1) Covalent bonds are converted to ionic bonds. (2) Isotopes are converted to isomers. (3) Temperature is converted to mass. (4) Matter is converted to energy
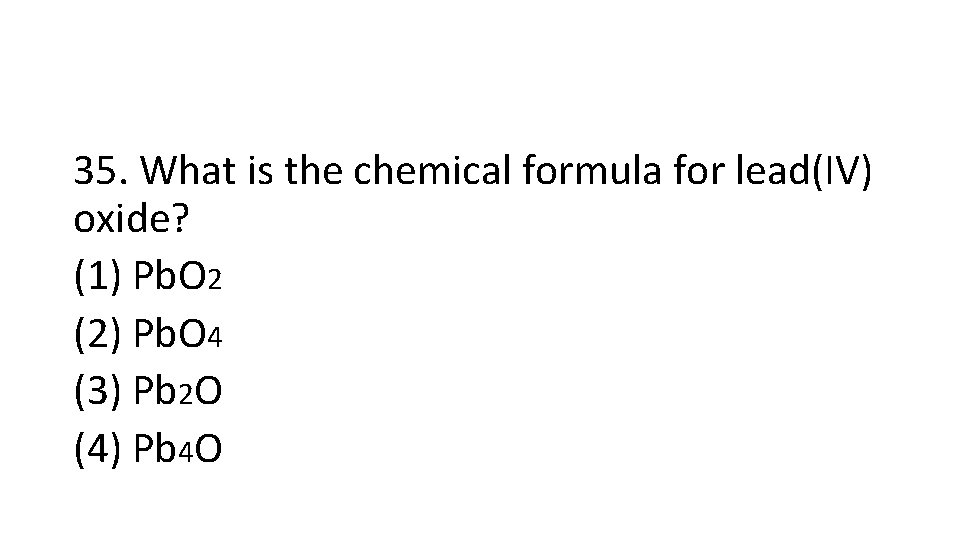
35. What is the chemical formula for lead(IV) oxide? (1) Pb. O 2 (2) Pb. O 4 (3) Pb 2 O (4) Pb 4 O

37 What is the percent composition by mass of nitrogen in (NH 4)2 CO 3 (gram-formula mass = 96. 0 g/mol)? (1) 14. 6% (2) 29. 2% (3) 58. 4% (4) 87. 5%

43. Which aqueous solution has the highest boiling point at standard pressure? (1)1. 0 M KCl(aq) (2)1. 0 M Ca. Cl 2(aq) (3)2. 0 M KCl(aq) (4)2. 0 M Ca. Cl 2(aq)

46. Which volume of 0. 600 M H 2 SO 4(aq) exactly neutralizes 100. milliliters of 0. 300 M Ba(OH)2(aq)? (1) 25. 0 m. L (2) 50. 0 m. L (3) 100. m. L (4) 200. m. L

50. Which type of organic reaction produces both water and carbon dioxide? (1) addition (2) combustion (3) esterification (4) fermentation
![51 Draw a Lewis electrondot diagram for a chloride ion Cl 1 57 State 51. Draw a Lewis electron-dot diagram for a chloride ion, Cl [1] 57. State](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-18.jpg)
51. Draw a Lewis electron-dot diagram for a chloride ion, Cl [1] 57. State the type of intermolecular force responsible for the unusual boiling point of H 2 O(ℓ) at standard pressure. [1]
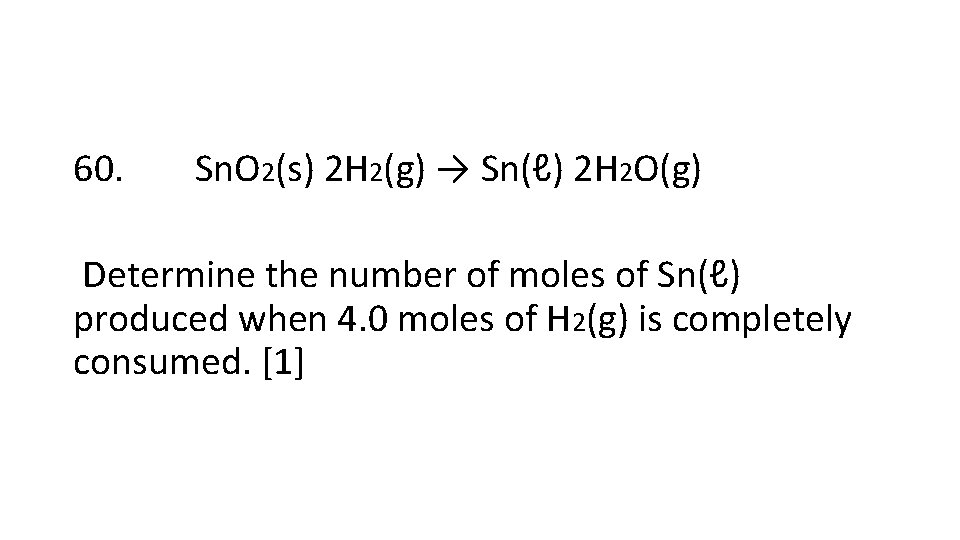
60. Sn. O 2(s) 2 H 2(g) → Sn(ℓ) 2 H 2 O(g) Determine the number of moles of Sn(ℓ) produced when 4. 0 moles of H 2(g) is completely consumed. [1]

Potassium phosphate, K 3 PO 4, is a source of dietary potassium found in a popular cereal. 66. Identify two types of chemical bonding in the source of dietary potassium in this cereal. [1] 73 Explain, in terms of electrons, how a strontium salt emits colored light. [1]
![78 State the purpose of the salt bridge in the voltaic cell 1 79 78. State the purpose of the salt bridge in the voltaic cell. [1] 79.](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-21.jpg)
78. State the purpose of the salt bridge in the voltaic cell. [1] 79. Complete and balance the half-reaction equation in your answer booklet for the oxidation of the Zn(s) that occurs in the voltaic cell. [1] 79. Zn(s) → _______ + _______

80. Compare the activities of the two metals used by the student for constructing the voltaic cell. [1] 81. Identify one item of laboratory equipment required to build an electrolytic cell that is not included in the list. [1]
![84 Determine the number of neutrons in an atom of U233 1 85 Identify 84. Determine the number of neutrons in an atom of U-233. [1] 85. Identify](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-23.jpg)
84. Determine the number of neutrons in an atom of U-233. [1] 85. Identify the type of nuclear reaction that occurs when an alpha or a beta particle is spontaneously emitted by a radioactive isotope. [1]

Answers 4. 9. 11. 13. 17. 18. 19. 4 2 3 1 3 4 3 24. 22. 3 27. 30. 35. 37. 1 43. 4 4 4 1 2 46. 50. 2 2
![Answers 51 Teacher will show on board 57 1 Allow 1 credit Acceptable responses Answers 51. Teacher will show on board. 57 [1] Allow 1 credit. Acceptable responses](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-25.jpg)
Answers 51. Teacher will show on board. 57 [1] Allow 1 credit. Acceptable responses include, but are not limited to: hydrogen bonding, H - bonding or dipole-dipole. 60 [1] Allow 1 credit for 2. 0 mol. Significant figures do not need to be shown.
![Answers 66 1 Allow 1 credit Acceptable responses include but are not limited to Answers 66 [1] Allow 1 credit. Acceptable responses include, but are not limited to:](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-26.jpg)
Answers 66 [1] Allow 1 credit. Acceptable responses include, but are not limited to: polar covalent and ionic, ionic and covalent or polar and ionic. 73 [1] Allow 1 credit. Acceptable responses include, but are not limited to: When strontium electrons in an excited state move to a lower energy state, specific amounts of energy are emitted. Energy is emitted when electrons in higher electron shells move to lower electron shells. Light of specific wavelengths is emitted when electrons fall to lower energy levels. Electrons move from higher shells to lower shells.
![Answers 78 1 Allow 1 credit Acceptable responses include but are not limited to Answers 78 [1] Allow 1 credit. Acceptable responses include, but are not limited to:](https://slidetodoc.com/presentation_image_h2/da27f124c896e0057481fb9ff1c305db/image-27.jpg)
Answers 78 [1] Allow 1 credit. Acceptable responses include, but are not limited to: The salt bridge allows ions to migrate between the half-cells. Electrical neutrality of the solutions is maintained. The purpose is to prevent polarization. allows charge to flow 79. Teacher will show half reaction on board.

Answers 80. Allow 1 credit. Acceptable responses include, but are not limited to: Zn is more active than Cu or Cu is located below Zn on Table J. 81. Allow 1 credit. Acceptable responses include, but are not limited to: battery, external power source, source of electricity. 84. Allow 1 credit for 141. 85 [1] Allow 1 credit. Acceptable responses include, but are not limited to: natural transmutation, nuclear decay, radioactive decay or decay.
 June 2010 chemistry regents
June 2010 chemistry regents Jan 2018 chem regents
Jan 2018 chem regents Nysedregents
Nysedregents Checkpoint
Checkpoint Solutions chemistry regents questions
Solutions chemistry regents questions Chemistry regents bonding questions
Chemistry regents bonding questions June 2007 physics regents answers
June 2007 physics regents answers June 21 2019 geometry regents answers
June 21 2019 geometry regents answers Mitsl
Mitsl Chemistry regents 2011
Chemistry regents 2011 Chemistry regents conversion chart
Chemistry regents conversion chart Nysedregents
Nysedregents Regents chemistry midterm
Regents chemistry midterm Spanish flacs exam 2018 answers
Spanish flacs exam 2018 answers Grade 7 life orientation term 1 notes
Grade 7 life orientation term 1 notes Flacs writing rubric
Flacs writing rubric Ohm's law worksheet regents physics
Ohm's law worksheet regents physics What were two indirect results of the crusades
What were two indirect results of the crusades Byzantine empire regents questions
Byzantine empire regents questions Decolonization regents questions
Decolonization regents questions Projectile motion regents questions
Projectile motion regents questions Characteristics of life regents questions
Characteristics of life regents questions Endocrine system regents questions
Endocrine system regents questions Endocrine system regents questions
Endocrine system regents questions Hospitality hsc exam
Hospitality hsc exam Welcome back your school missed you
Welcome back your school missed you Paylocity
Paylocity Esterman visual field dvla
Esterman visual field dvla Gsil concord nh
Gsil concord nh