Most Missed Questions June 2009 1 State in




- Slides: 12

Most Missed Questions June 2009

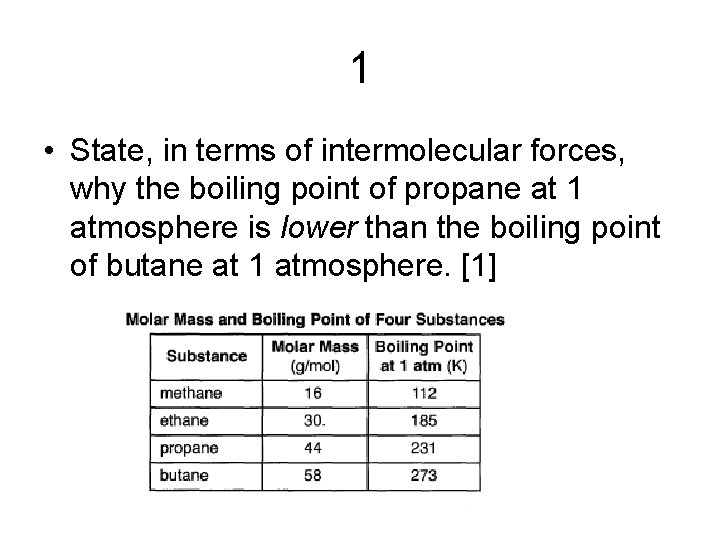
1 • State, in terms of intermolecular forces, why the boiling point of propane at 1 atmosphere is lower than the boiling point of butane at 1 atmosphere. [1]

2 • Which element is composed of molecules that each contain a multiple covalent bond? • 1 chlorine • 2 fluorine • 3 hydrogen • 4 nitrogen

3. • A beta particle may be spontaneously emitted from • 1 a ground-state electron • 2 a stable nucleus • 3 an excited electron • 4 an unstable nucleus

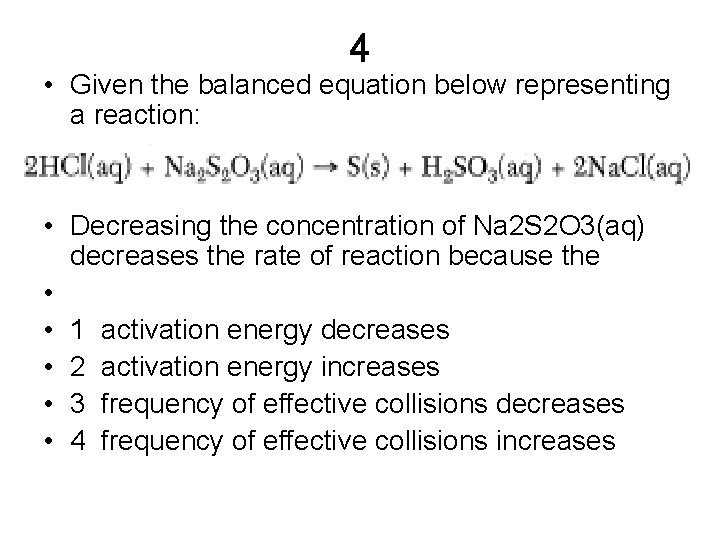
4 • Given the balanced equation below representing a reaction: • Decreasing the concentration of Na 2 S 2 O 3(aq) decreases the rate of reaction because the • • 1 activation energy decreases • 2 activation energy increases • 3 frequency of effective collisions decreases • 4 frequency of effective collisions increases

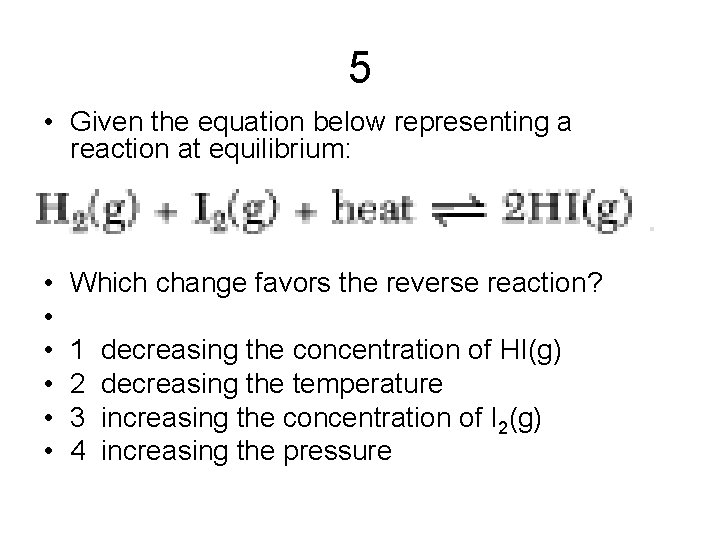
5 • Given the equation below representing a reaction at equilibrium: • • • Which change favors the reverse reaction? 1 decreasing the concentration of HI(g) 2 decreasing the temperature 3 increasing the concentration of I 2(g) 4 increasing the pressure

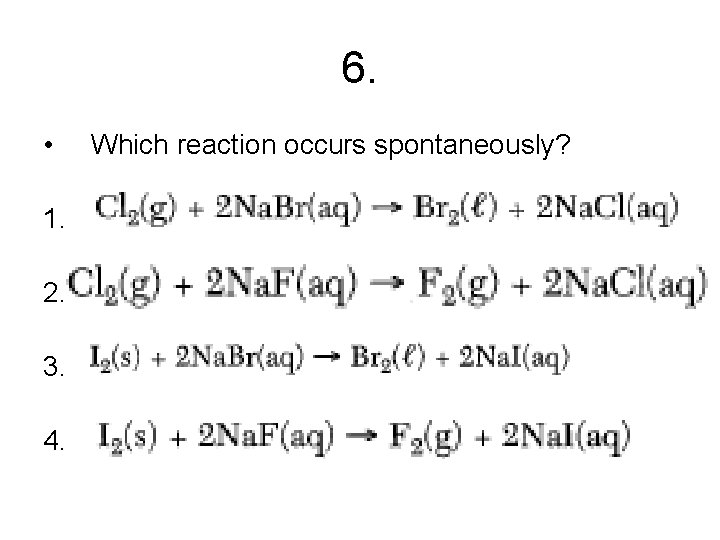
6. • Which reaction occurs spontaneously? 1. 2. 3. 4.

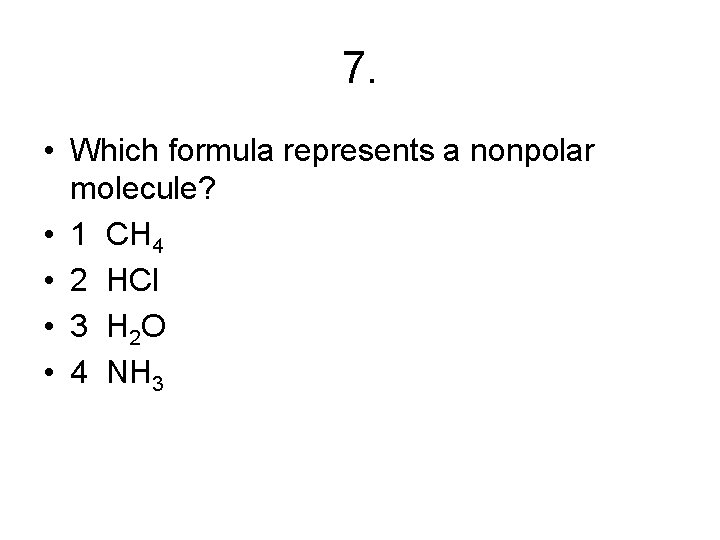
7. • Which formula represents a nonpolar molecule? • 1 CH 4 • 2 HCl • 3 H 2 O • 4 NH 3

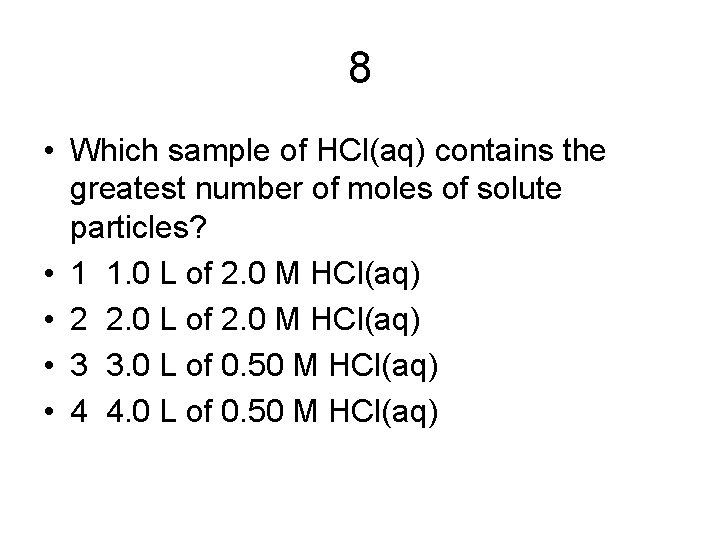
8 • Which sample of HCl(aq) contains the greatest number of moles of solute particles? • 1 1. 0 L of 2. 0 M HCl(aq) • 2 2. 0 L of 2. 0 M HCl(aq) • 3 3. 0 L of 0. 50 M HCl(aq) • 4 4. 0 L of 0. 50 M HCl(aq)

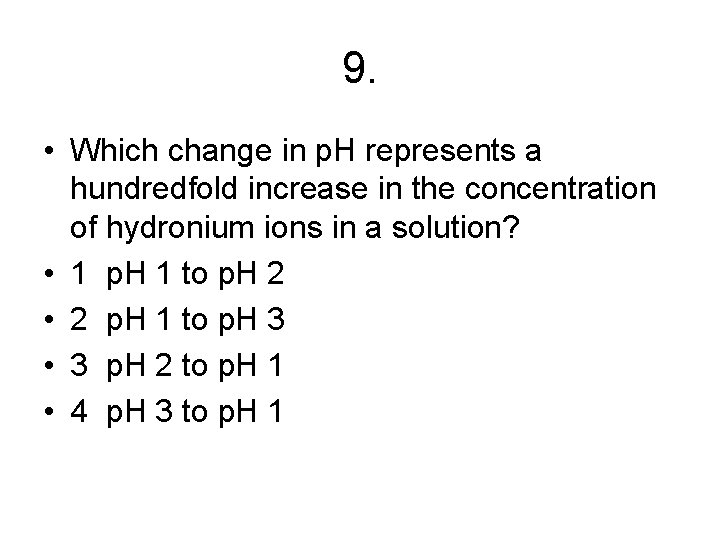
9. • Which change in p. H represents a hundredfold increase in the concentration of hydronium ions in a solution? • 1 p. H 1 to p. H 2 • 2 p. H 1 to p. H 3 • 3 p. H 2 to p. H 1 • 4 p. H 3 to p. H 1

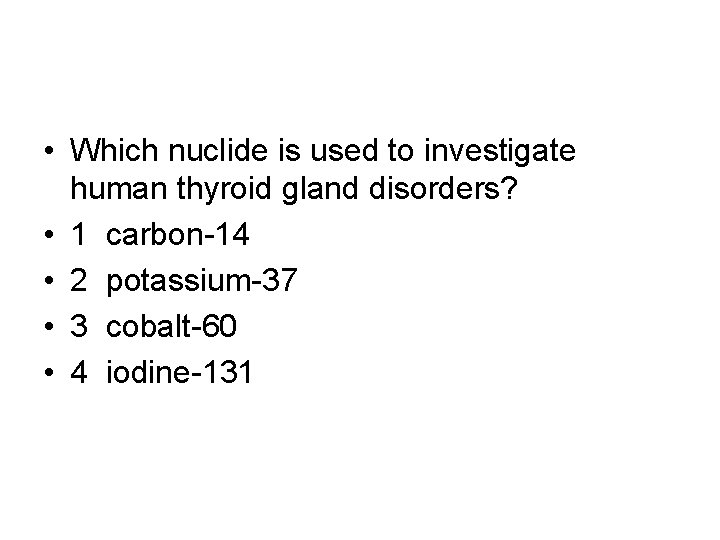
• Which nuclide is used to investigate human thyroid gland disorders? • 1 carbon-14 • 2 potassium-37 • 3 cobalt-60 • 4 iodine-131

Answers 1. 2. 4 3. 4 4. 3 5. 2 6. 1 7. 1 8. 2. 9. 4 10. 4
 Welcome back your school missed you
Welcome back your school missed you Paylocity forgot password
Paylocity forgot password Ocular hypertension dvla
Ocular hypertension dvla Gsil missed punch
Gsil missed punch We missed you welcome back
We missed you welcome back Romeo and juliet script oh romeo i've missed you
Romeo and juliet script oh romeo i've missed you Romeo and juliet quotes oh romeo
Romeo and juliet quotes oh romeo Missed connections arlington va
Missed connections arlington va Gsil missed punch form
Gsil missed punch form Workforce timekeeper
Workforce timekeeper Cisco ip phone 7965 voicemail
Cisco ip phone 7965 voicemail Most general to most specific classification
Most general to most specific classification Most general to most specific classification
Most general to most specific classification























