Most missed questions June 2012 12 What is

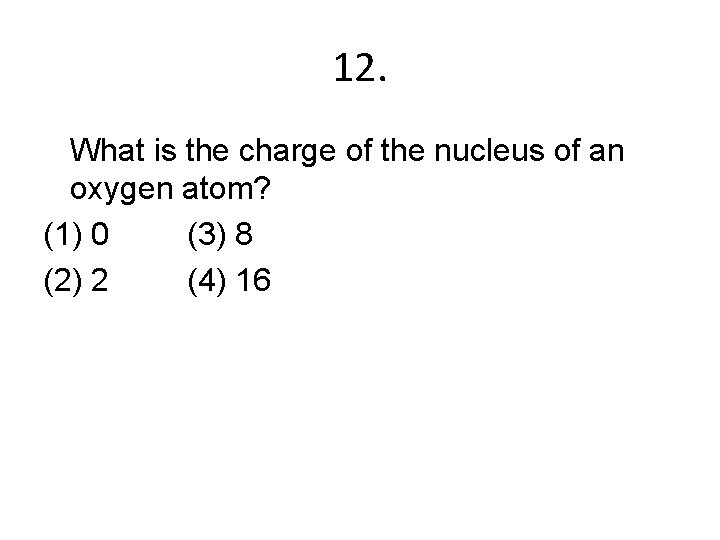







- Slides: 17

Most missed questions June 2012
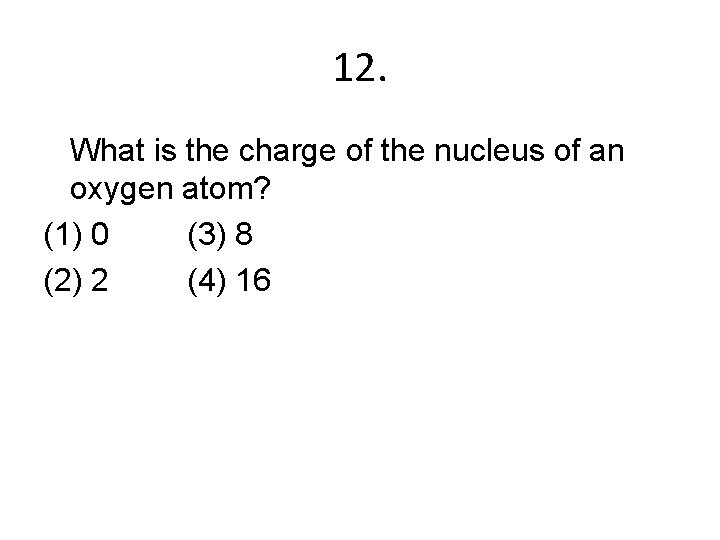
12. What is the charge of the nucleus of an oxygen atom? (1) 0 (3) 8 (2) 2 (4) 16

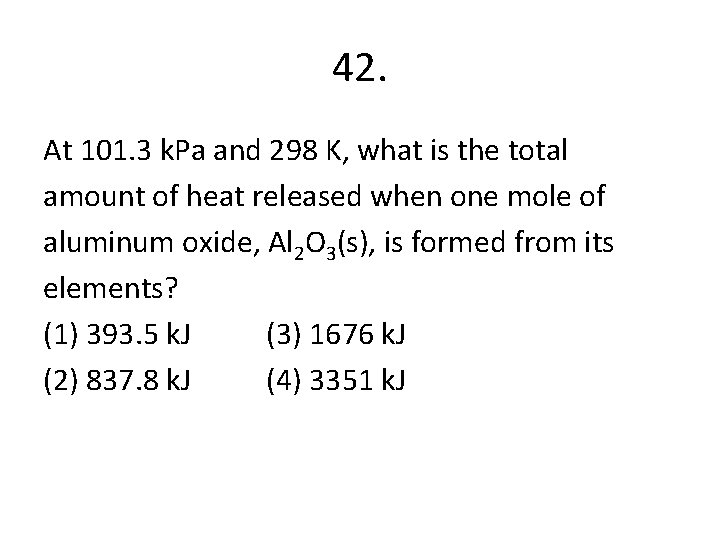
42. At 101. 3 k. Pa and 298 K, what is the total amount of heat released when one mole of aluminum oxide, Al 2 O 3(s), is formed from its elements? (1) 393. 5 k. J (3) 1676 k. J (2) 837. 8 k. J (4) 3351 k. J

47 • Which ion is most easily reduced? • (l ) Zn 2+ (2) Mg 2+ (3) Co 2+ (4) Ca 2+

41 At 50. °C and standard pressure, intermolecular forces of attraction are strongest in a sample of (1) ethanoic acid (3) propanone (2) ethanol (4) water ++Most missed question on exam++ Hint use Table H, the lowest vapor pressure has the strongest IMFs

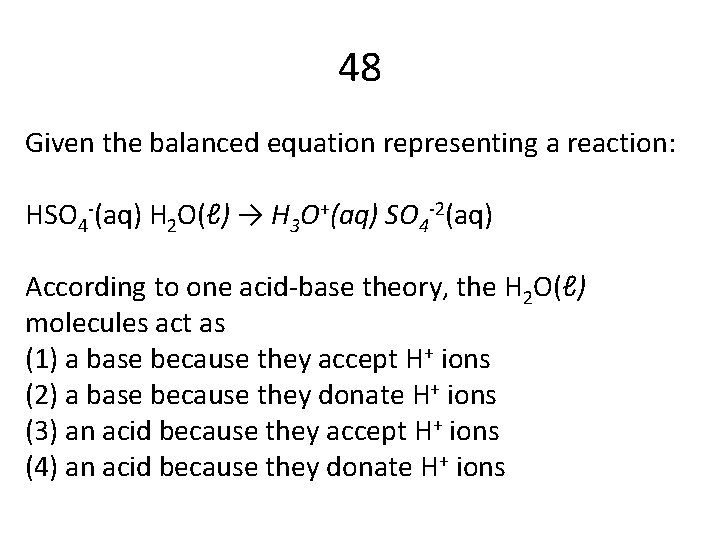
48 Given the balanced equation representing a reaction: HSO 4 -(aq) H 2 O(ℓ) → H 3 O+(aq) SO 4 -2(aq) According to one acid-base theory, the H 2 O(ℓ) molecules act as (1) a base because they accept H+ ions (2) a base because they donate H+ ions (3) an acid because they accept H+ ions (4) an acid because they donate H+ ions

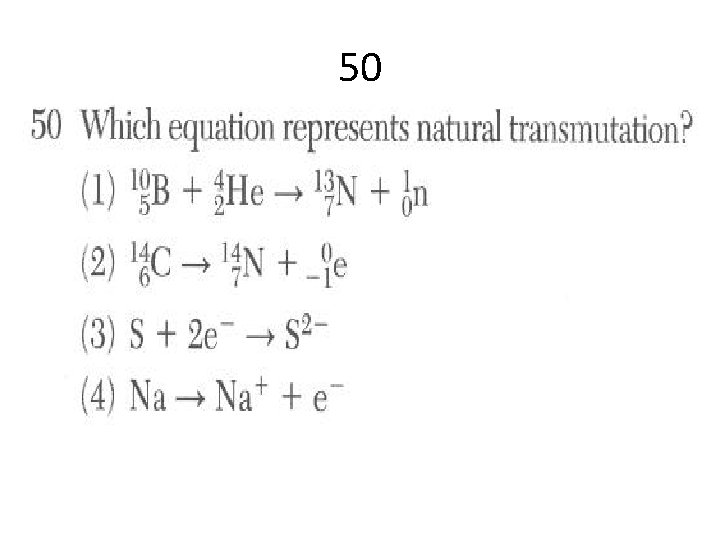
29 Compared to the mass and the penetrating power of an alpha particle, a beta particle has (1) less mass and greater penetrating power (2) less mass and less penetrating power (3) more mass and greater penetrating power (4) more mass and less penetrating power

50

9 A compound is broken down by chemical means during (1) chromatography (3) electrolysis (2) distillation (4) filtration

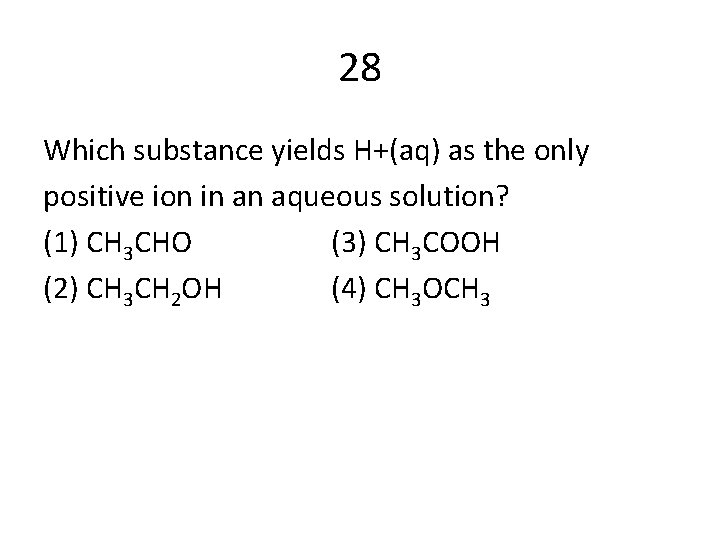
28 Which substance yields H+(aq) as the only positive ion in an aqueous solution? (1) CH 3 CHO (3) CH 3 COOH (2) CH 3 CH 2 OH (4) CH 3 OCH 3

14 To break a chemical bond, energy must be (1) absorbed (3) produced (2) destroyed (4) released

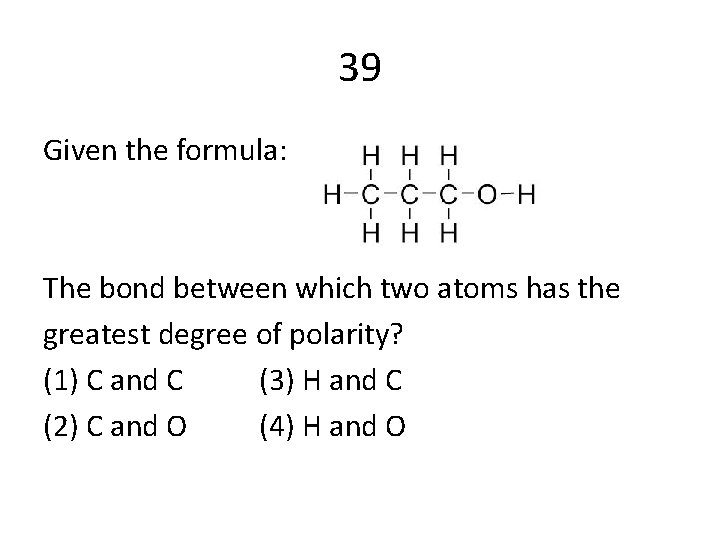
39 Given the formula: The bond between which two atoms has the greatest degree of polarity? (1) C and C (3) H and C (2) C and O (4) H and O

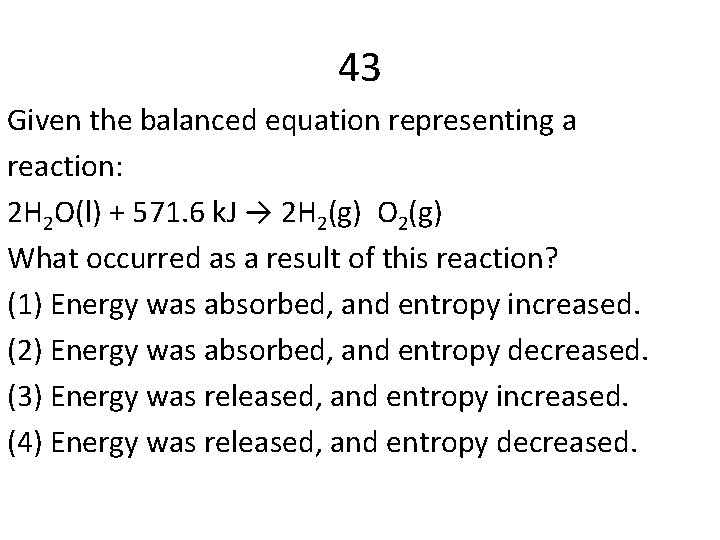
43 Given the balanced equation representing a reaction: 2 H 2 O(l) + 571. 6 k. J → 2 H 2(g) O 2(g) What occurred as a result of this reaction? (1) Energy was absorbed, and entropy increased. (2) Energy was absorbed, and entropy decreased. (3) Energy was released, and entropy increased. (4) Energy was released, and entropy decreased.

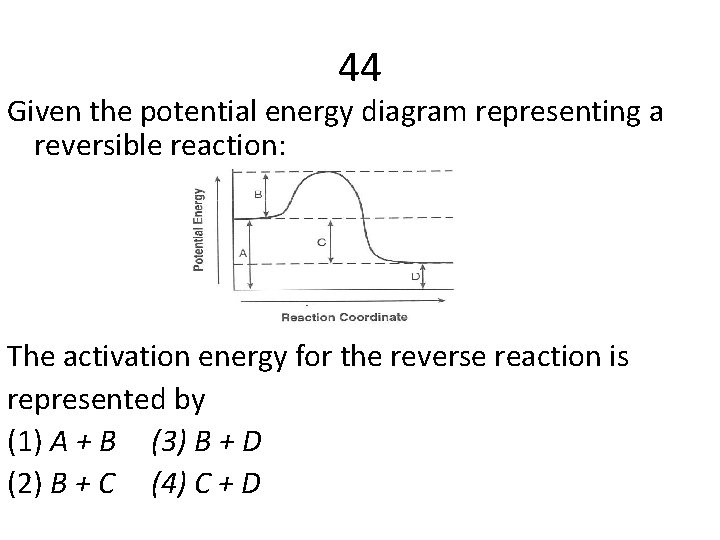
44 Given the potential energy diagram representing a reversible reaction: The activation energy for the reverse reaction is represented by (1) A + B (3) B + D (2) B + C (4) C + D

4 Which element is a liquid at 305 K and 1. 0 atmosphere? (1) magnesium (2) fluorine (3) gallium (4) iodine

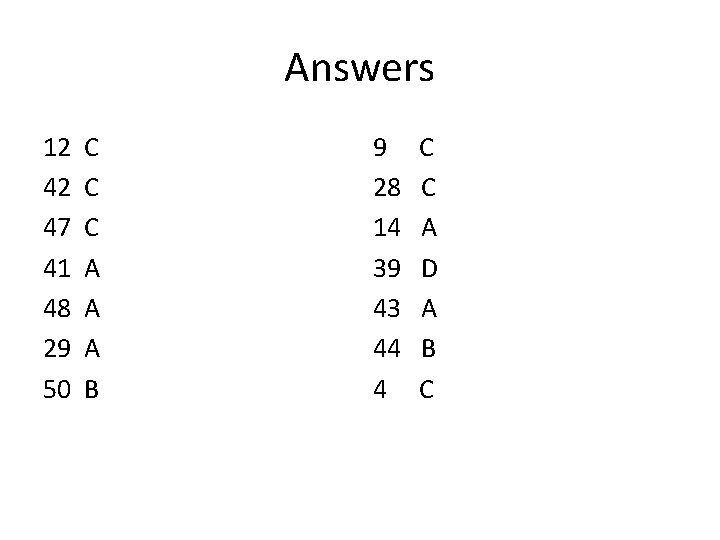
Answers 12 C 47 C 41 A 48 A 29 A 50 B 9 C 28 C 14 A 39 D 43 A 44 B 4 C

• • • Constructed response questions to consider: 78 55 73 65 71 74 64 57 68
 Elephant riding in phuket icfes respuestas
Elephant riding in phuket icfes respuestas Nysedregents
Nysedregents Welcome back we've missed you
Welcome back we've missed you Paylocity training
Paylocity training Esterman efficiency score driving
Esterman efficiency score driving Gsil concord nh
Gsil concord nh We missed you welcome back
We missed you welcome back Romeo romeo where art thou romeo script
Romeo romeo where art thou romeo script Romeo wherefore art thou
Romeo wherefore art thou Missed connections arlington va
Missed connections arlington va Gsil missed punch form
Gsil missed punch form Kronos workforce timekeeper
Kronos workforce timekeeper Missed call from nnn
Missed call from nnn Most general to most specific classification
Most general to most specific classification Most general to most specific classification
Most general to most specific classification In the name of allah most gracious most merciful
In the name of allah most gracious most merciful In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful In the name of allah the most beneficent the most merciful
In the name of allah the most beneficent the most merciful