Molten Salt Method of Preparation and Optimization of


![X-Ray Powder Diffraction • Determination of crystal structures Sample 5 [5] M. V. Reddy, X-Ray Powder Diffraction • Determination of crystal structures Sample 5 [5] M. V. Reddy,](https://slidetodoc.com/presentation_image_h/8e4b6a3de3ce6d14eceea06854f36e94/image-15.jpg)

- Slides: 29

Molten Salt Method of Preparation and Optimization of Ti. O 2 Phases Chan Tze Yang, Aloysius 1, 2, M. V. Reddy 2, 3*, S. Adams 3 and B. V. R. Chowdari 2 1 2 SRP Student, Hwa Chong Institution, 661, Bukit Timah Road Singapore 269734 Department of Physics, Faculty of Science, National University of Singapore, 2 Science Drive 3, Singapore 117542 3 Department of Materials Science and Engineering, National University of Singapore, Singapore 117546 *Corresponding main mentor’s e-mail address: phymvvr@nus. edu. sg ; msemvvr@nus. edu. sg http: //www. researcherid. com/rid/B-3524 -2010 http: //scholar. google. com. sg/citations? user=p. WKr 2 M 0 AAAAJ&hl=en 1

2

High Costs Low Charge Rates Low Thermal Stability Low Theoretical Capacity Graphite 3

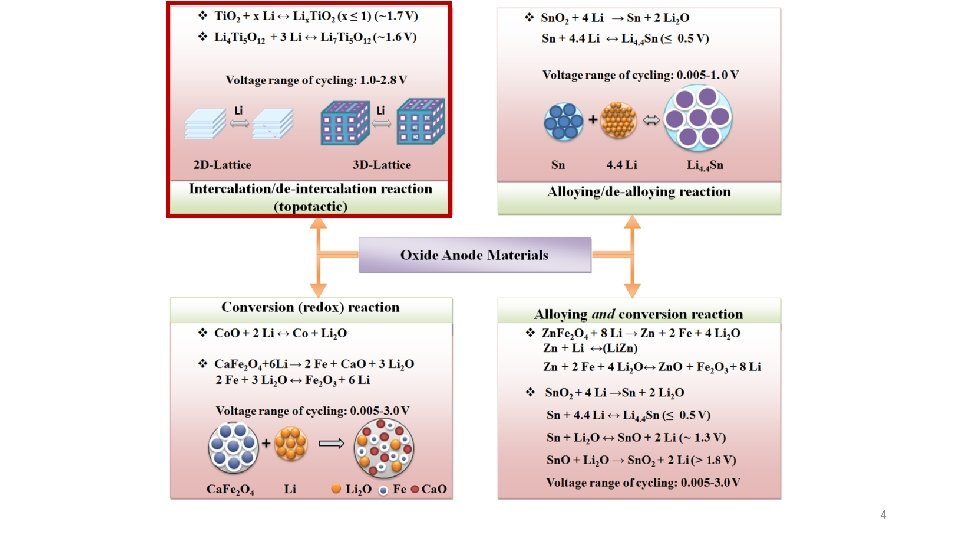
4

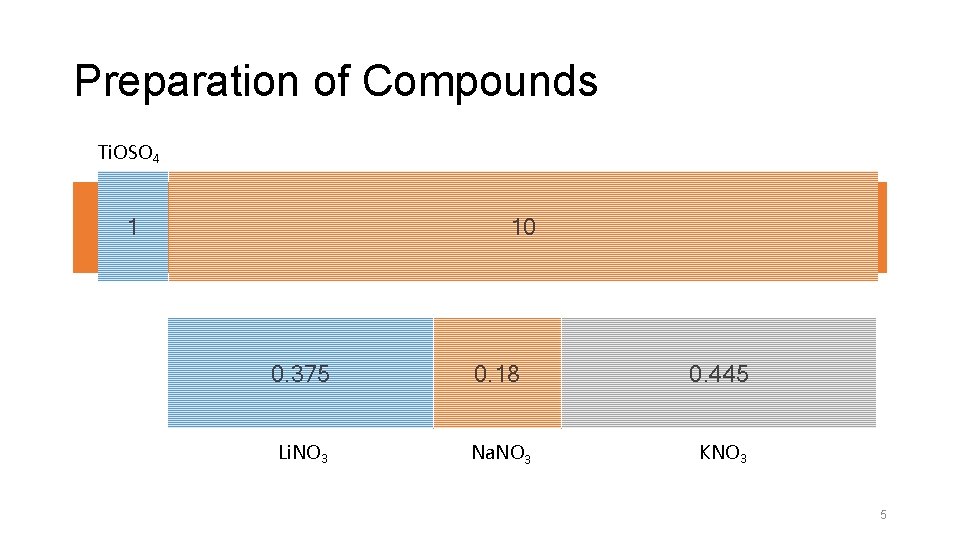
Preparation of Compounds Ti. OSO 4 1 Molten Salt Method 10 0. 375 0. 18 0. 445 Li. NO 3 Na. NO 3 KNO 3 5

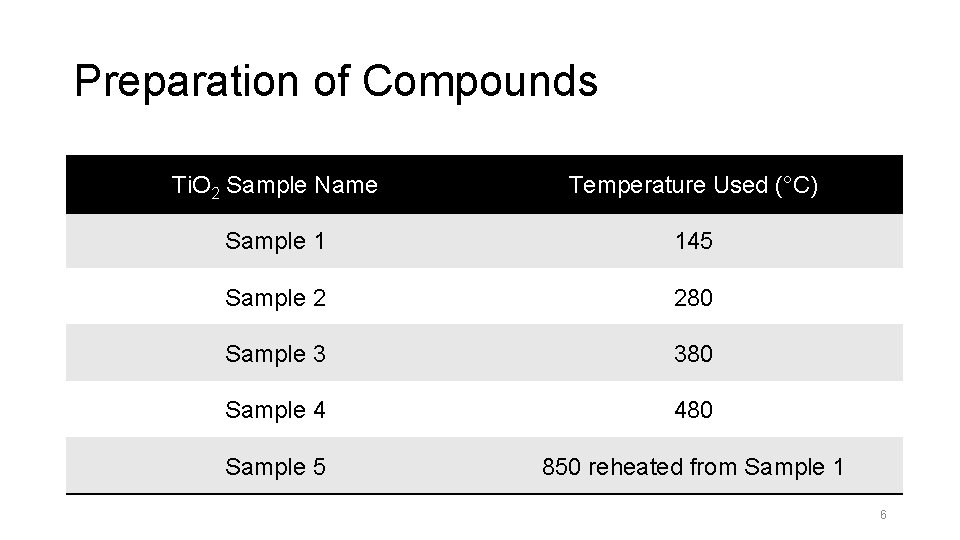
Preparation of Compounds Ti. O 2 Sample Name Temperature Used (°C) Sample 1 145 Sample 2 280 Sample 3 380 Sample 4 480 Sample 5 850 reheated from Sample 1 6

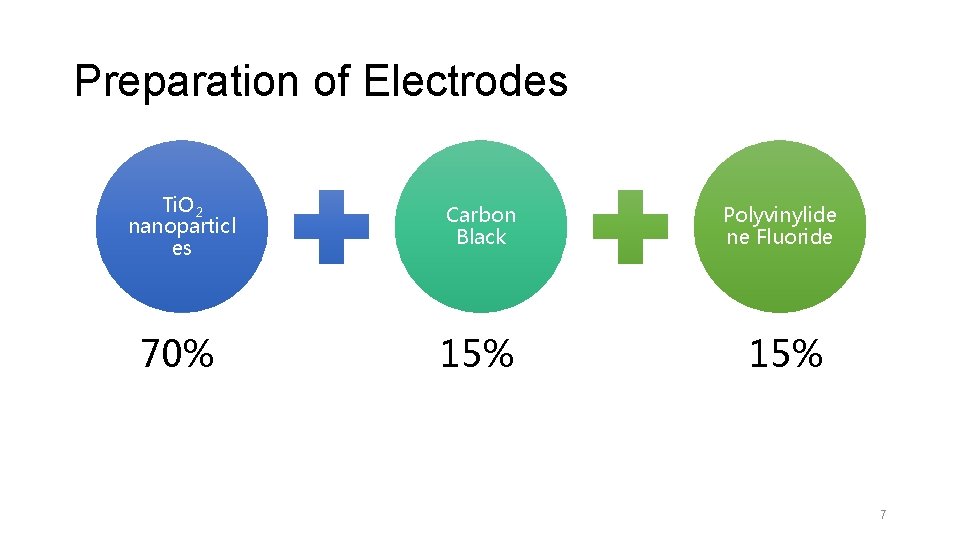
Preparation of Electrodes Ti. O 2 nanoparticl es Carbon Black Polyvinylide ne Fluoride 70% 15% 7

Preparation of Electrodes 8

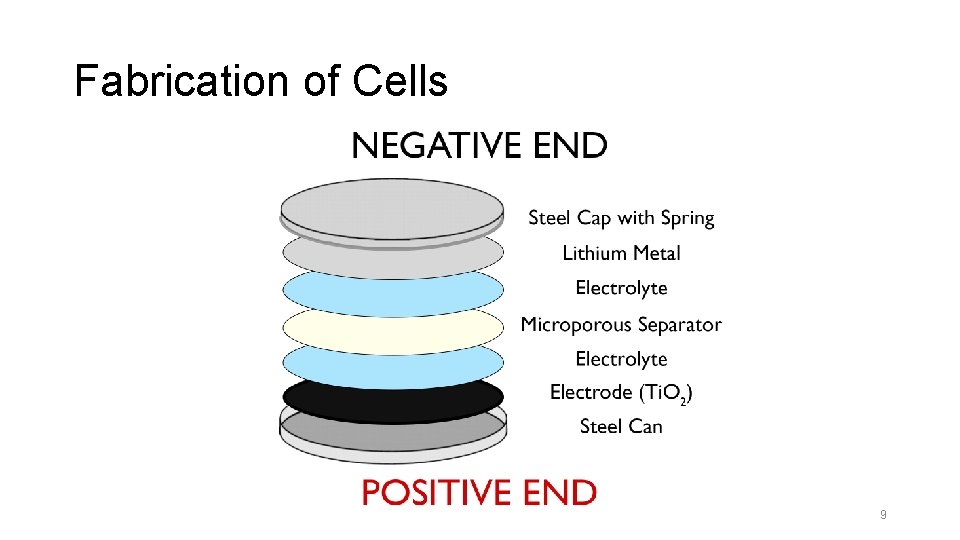
Fabrication of Cells 9

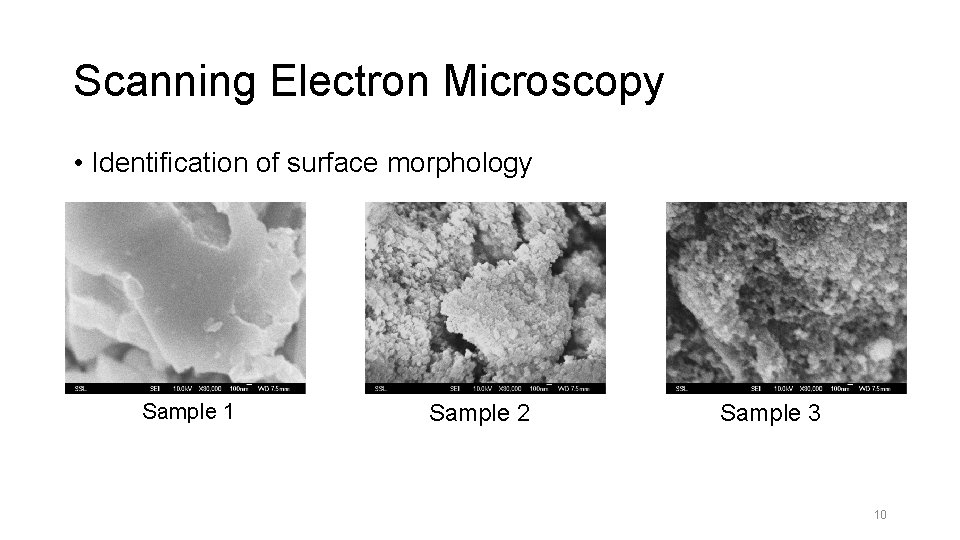
Scanning Electron Microscopy • Identification of surface morphology Sample 1 Sample 2 Sample 3 10

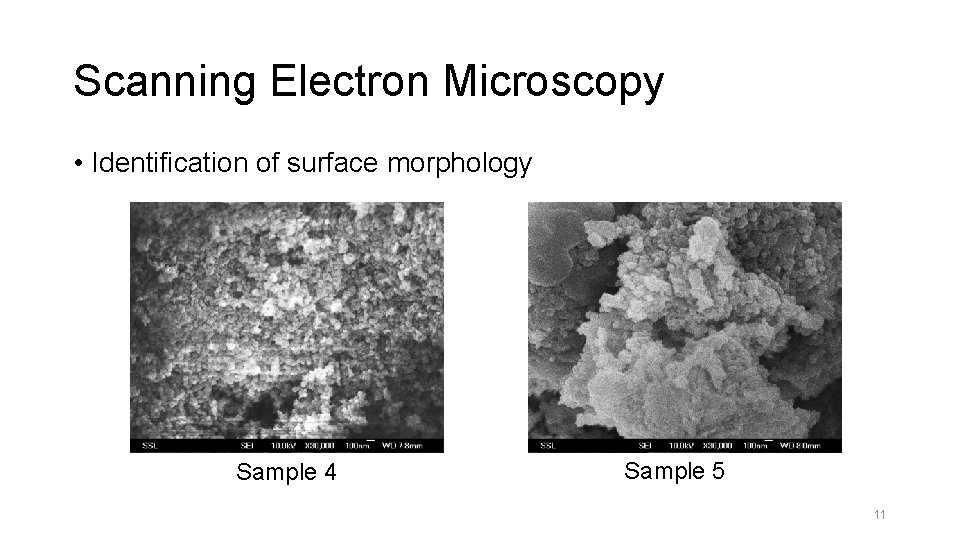
Scanning Electron Microscopy • Identification of surface morphology Sample 4 Sample 5 11

Scanning Electron Microscopy Spheric al 12

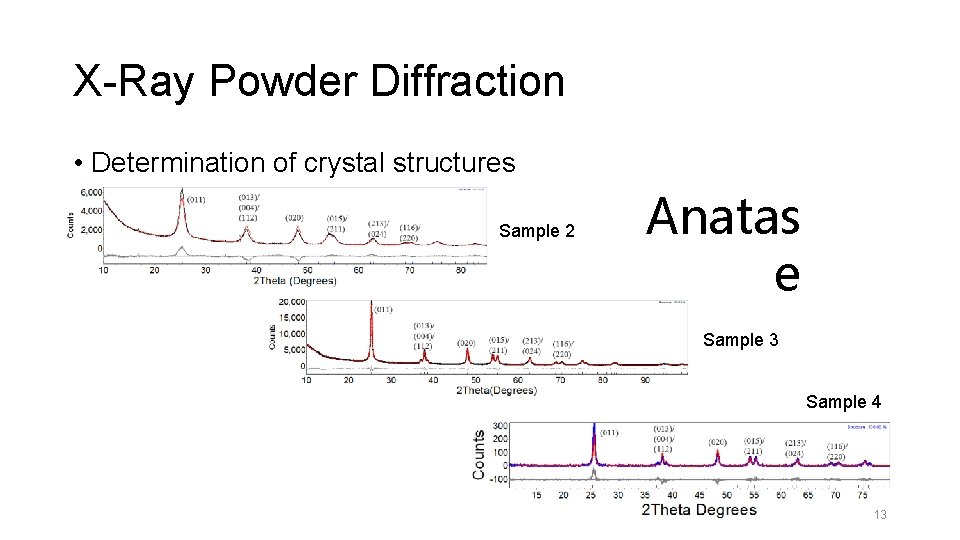
X-Ray Powder Diffraction • Determination of crystal structures Sample 2 Anatas e Sample 3 Sample 4 13

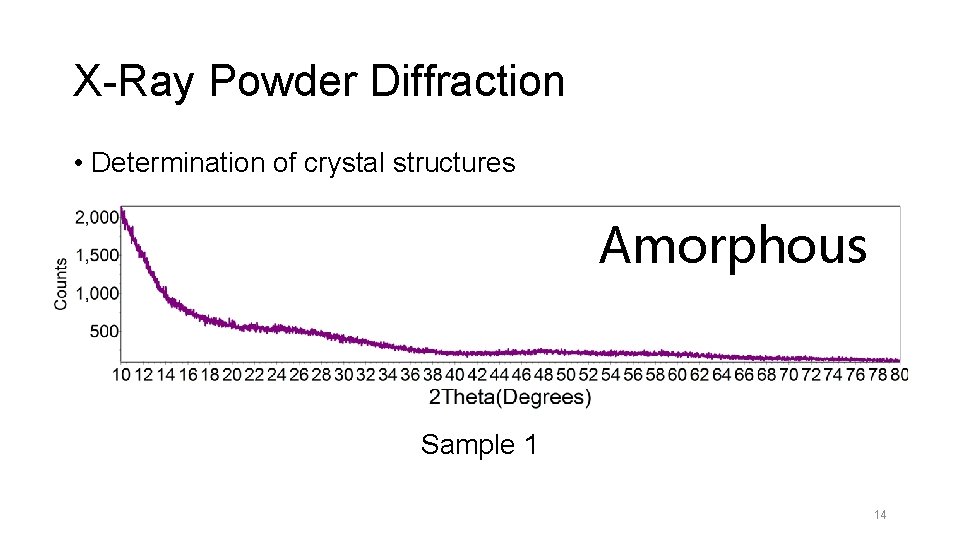
X-Ray Powder Diffraction • Determination of crystal structures Amorphous Sample 1 14
![XRay Powder Diffraction Determination of crystal structures Sample 5 5 M V Reddy X-Ray Powder Diffraction • Determination of crystal structures Sample 5 [5] M. V. Reddy,](https://slidetodoc.com/presentation_image_h/8e4b6a3de3ce6d14eceea06854f36e94/image-15.jpg)
X-Ray Powder Diffraction • Determination of crystal structures Sample 5 [5] M. V. Reddy, X. W. Valerie Teoh, T. B. Nguyen, Y. Y. Michelle Lim, and B. V. R. Chowdari. 2012. Effect of 0. 5 M Na. NO 3: 0. 5 MKNO 3 and 0. 88 M Li. NO 3: 0. 12 M Li. Cl Molten Salts, and Heat Treatment on Electrochemical Properties of Ti. O 2. Journal of The Electrochemical Society, 159 (6) A 762 A 769. Anatase 15

Cyclic Voltammetry • Investigate the redox behavior of Ti. O 2 in the electrolyte • Voltage Range: 1. 0 V – 2. 8 V • Scan Rate 0. 058 m. V/s 16

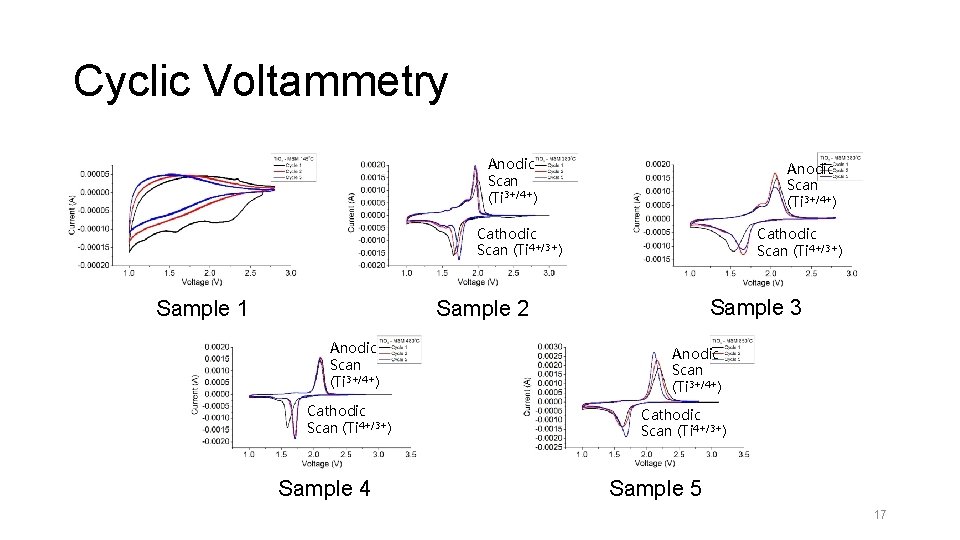
Cyclic Voltammetry Anodic Scan (Ti 3+/4+) Cathodic Scan (Ti 4+/3+) Sample 1 Cathodic Scan (Ti 4+/3+) Sample 3 Sample 2 Anodic Scan (Ti 3+/4+) Cathodic Scan (Ti 4+/3+) Sample 4 Anodic Scan (Ti 3+/4+) Cathodic Scan (Ti 4+/3+) Sample 5 17

Galvanostatic Cycling & Capacity Fading • Determine the suitability of using Ti. O 2 as anode material • Voltage Range: 1. 0 V – 2. 8 V • Current Rate: 33 m. A g-1 18

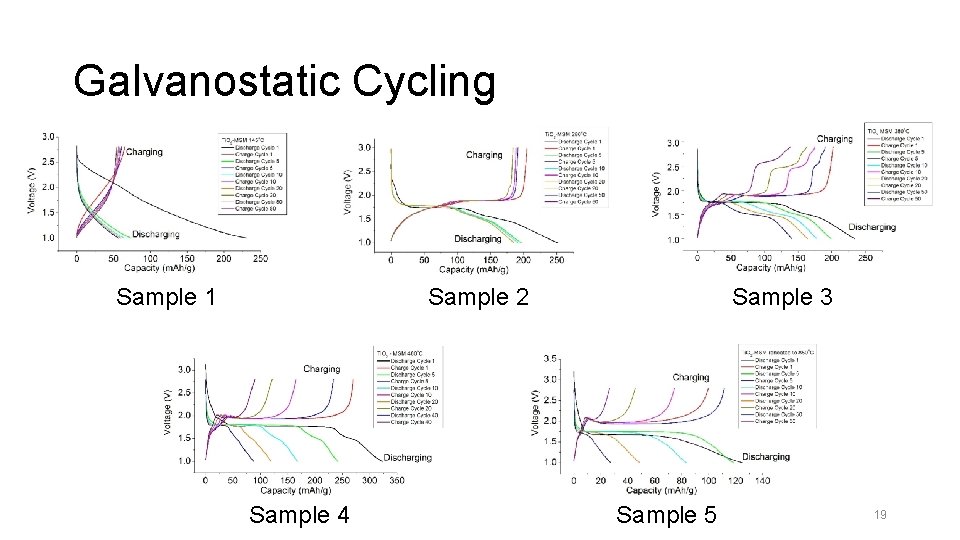
Galvanostatic Cycling Sample 1 Sample 3 Sample 2 Sample 4 Sample 5 19

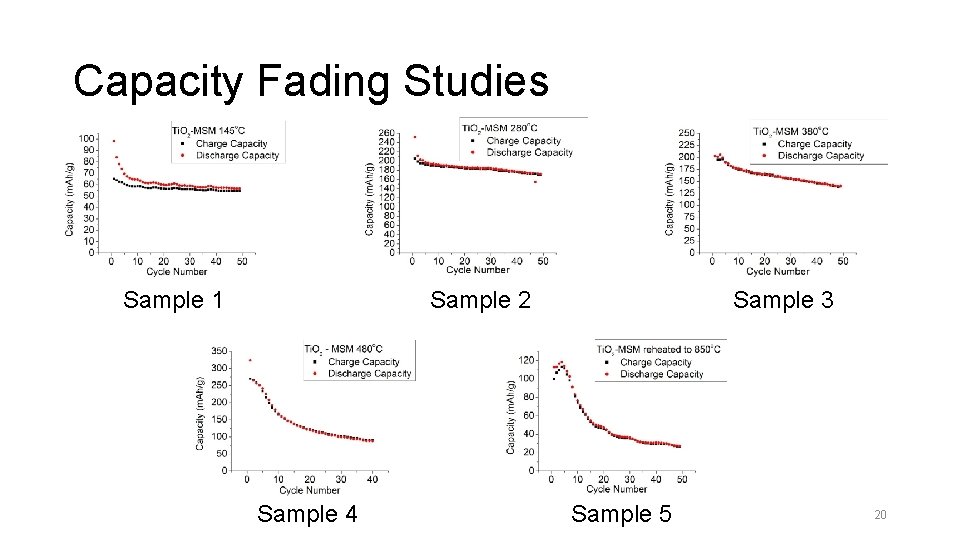
Capacity Fading Studies Sample 1 Sample 3 Sample 2 Sample 4 Sample 5 20

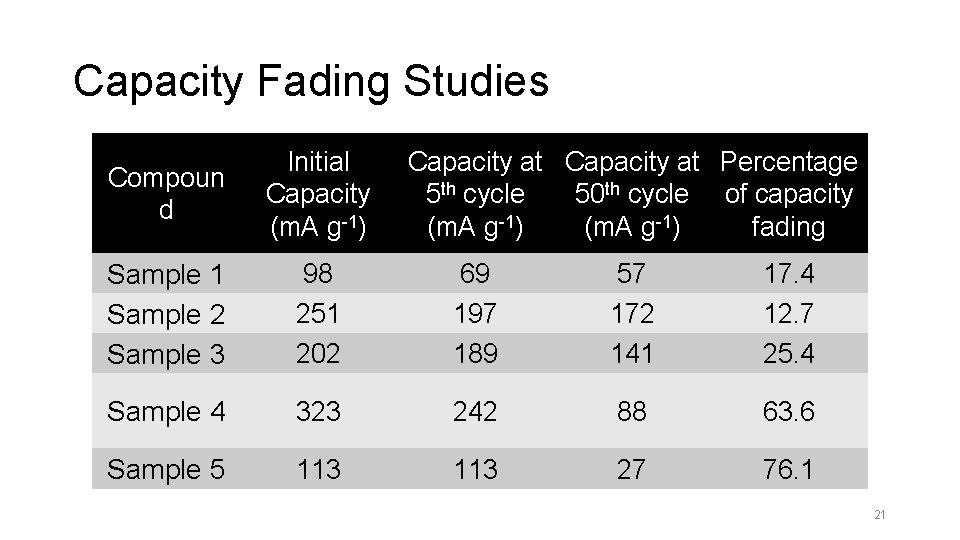
Capacity Fading Studies Compoun d Initial Capacity (m. A g-1) Capacity at Percentage 5 th cycle 50 th cycle of capacity (m. A g-1) fading Sample 1 Sample 2 Sample 3 98 251 202 69 197 189 57 172 141 17. 4 12. 7 25. 4 Sample 4 323 242 88 63. 6 Sample 5 113 27 76. 1 21

Electrochemical Impedance Spectroscopy • Determine the electrode kinetics within the cell • Voltage Range: 1. 0 V – 2. 8 V • Frequency Range: 0. 003 Hz – 180000 Hz • AC Amplitude: 10 m. V 22

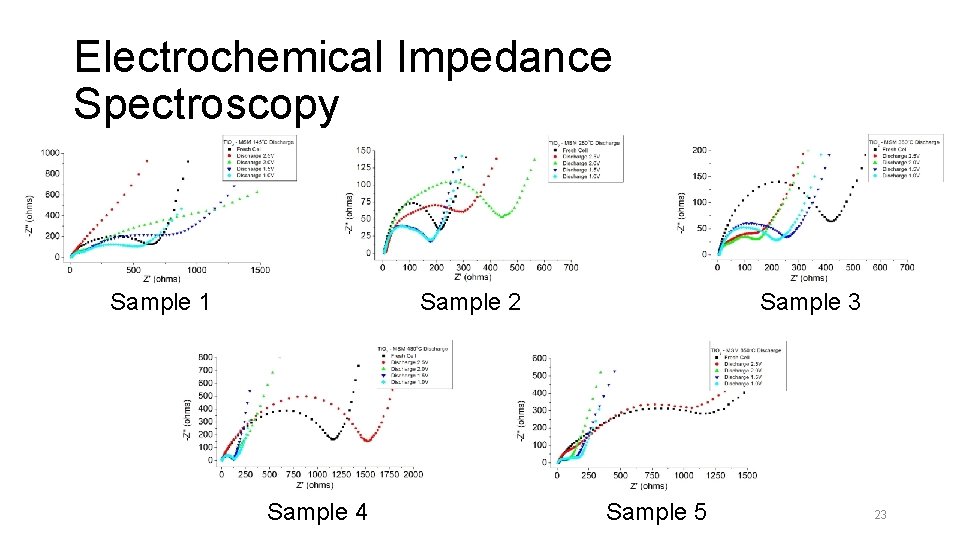
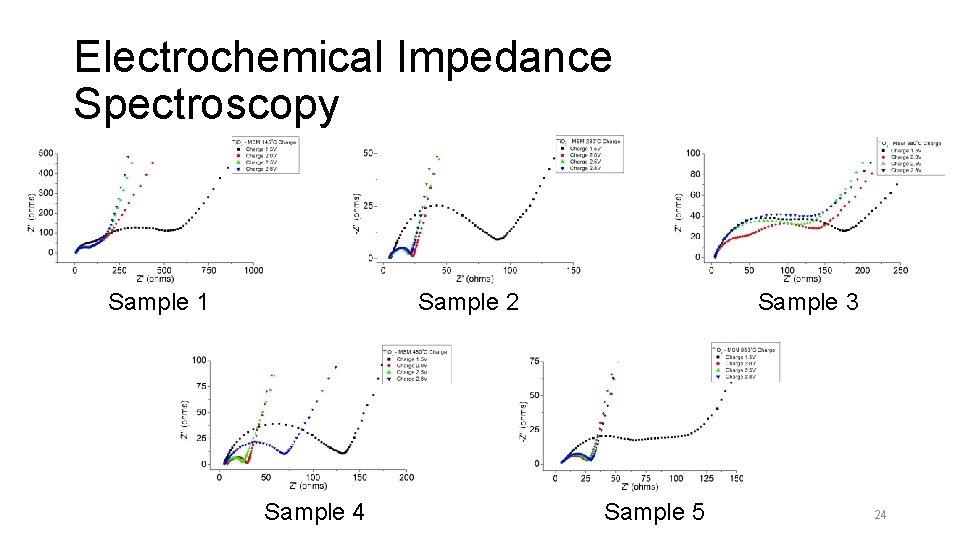
Electrochemical Impedance Spectroscopy Sample 1 Sample 3 Sample 2 Sample 4 Sample 5 23

Electrochemical Impedance Spectroscopy Sample 1 Sample 3 Sample 2 Sample 4 Sample 5 24

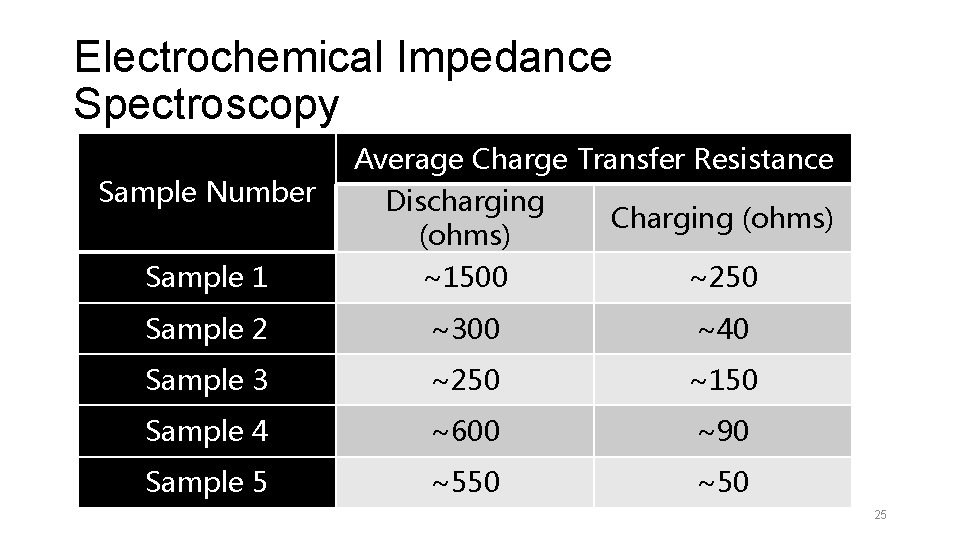
Electrochemical Impedance Spectroscopy Sample Number Average Charge Transfer Resistance Discharging (ohms) Charging (ohms) Sample 1 ~1500 ~250 Sample 2 ~300 ~40 Sample 3 ~250 ~150 Sample 4 ~600 ~90 Sample 5 ~550 ~50 25

Conclusions Amorpho us Ti. O 2 Reheat Anatase Ti. O 2 26

Conclusions Lower Production Temperature Better Electrochemical Properties 27

Conclusions Low Costs Of Production Environment al Friendliness High Capacity Retention Highly suitable alternative anode material in Li -ion Batteries 28

Thank You 29