Modelling of Kinetics in Multi Component MultiPhase Multi



![Calculation with improved database G(M 6 C)=G 0 -2600 [J/mol] M 6 C: 1, Calculation with improved database G(M 6 C)=G 0 -2600 [J/mol] M 6 C: 1,](https://slidetodoc.com/presentation_image/49ff1ef4610e64ca98707685ed5e888c/image-27.jpg)

- Slides: 32

Modelling of Kinetics in Multi. Component, Multi-Phase, Multi. Particle Systems: Application E. Kozeschnik J. Svoboda F. D. Fischer Institute for Materials Science, Welding and Forming, Graz University of Technology Materials Center Leoben, Austria Academy of Sciences, Brno, Czech Republic Institute of Metal Physics, University of Mining, Leoben , Austria Erich Schmid Institute of Materials Science, Austrian Academy of Sciences , Austria Institute of Mechanics, University of Mining, Leoben , Austria

Contents • • Model formulation Computer Implementation Algorithm flow-chart Application to – Nucleation, growth and coarsening of cementite in steel – TTP Diagram for gamma_prime in Ni-base – Complex experimental tool steel IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

The modeling team (2001 -2006). . . • J. Svoboda – Academy of Sciences, Czech Republic, CZ • F. D. Fischer – Institute of Mechanics, University of Leoben, A • E. Kozeschnik B. Sonderegger (2004 -) – Institute for Materials Science, Welding and Forming, Graz University of Technology, A Task: Model development and implementation for precipitation kinetics in multi-component, multi-phase, multi-particle systems IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Idea … • System with spherical precipitates of different size, composition and phase type in multi-component matrix. • Evolution equations from Onsager thermodynamic extremal principle: System develops with constrained maximum Gibbs Free Energy dissipation. IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

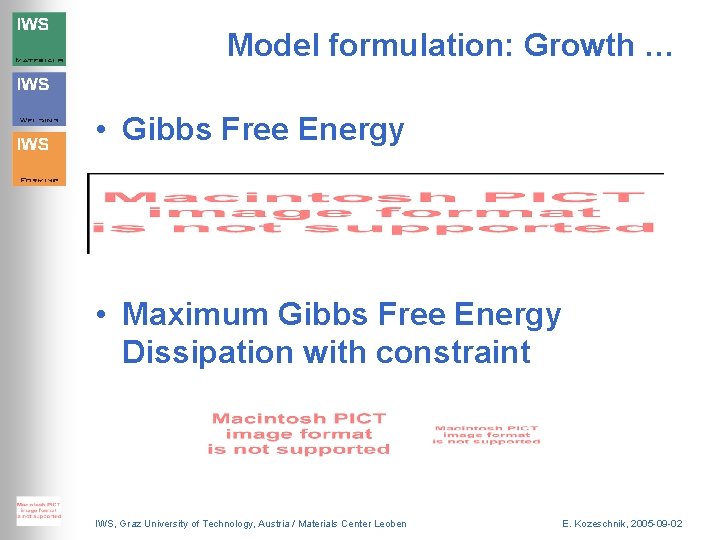
Model formulation: Growth … • Gibbs Free Energy • Maximum Gibbs Free Energy Dissipation with constraint IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

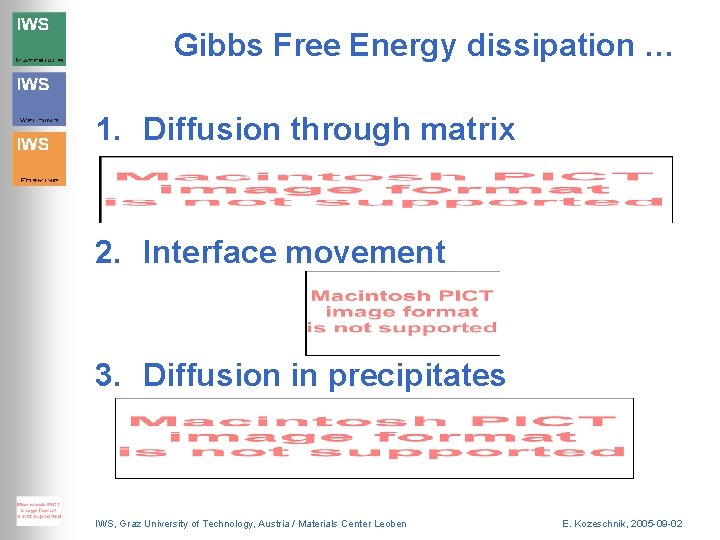
Gibbs Free Energy dissipation … 1. Diffusion through matrix 2. Interface movement 3. Diffusion in precipitates IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

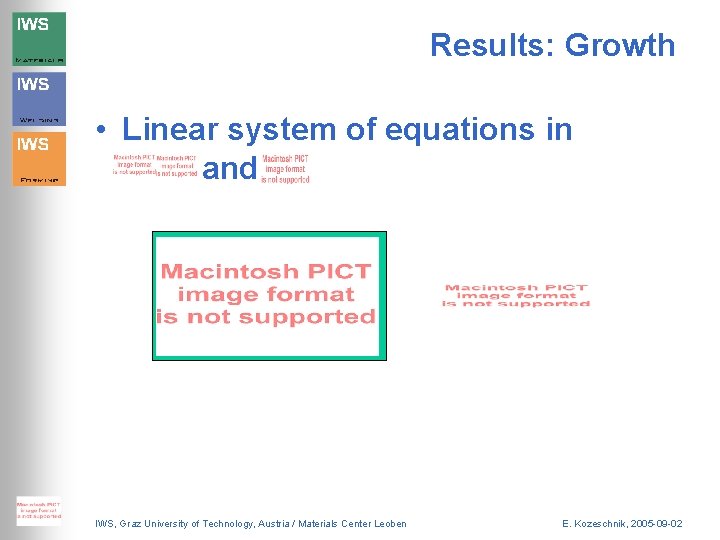
Results: Growth • Linear system of equations in , and : IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

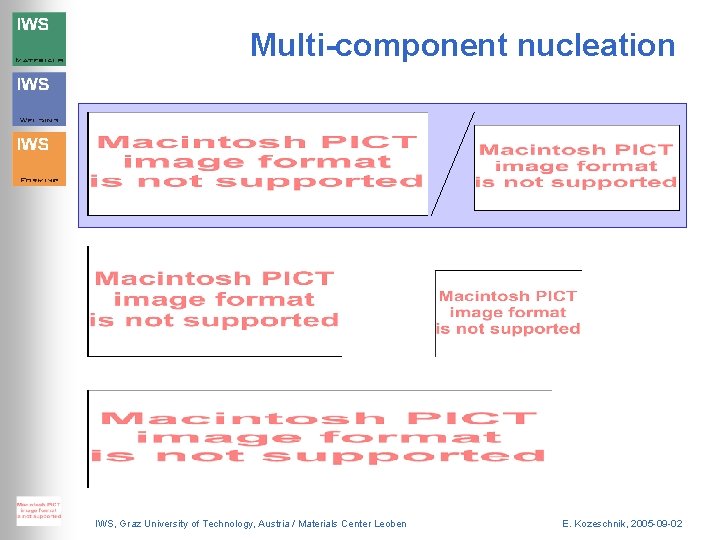
Multi-component nucleation IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Thermo-Kinetic software: Mat. Calc • Equilibrium (CALPHAD) • Diffusion (MOBILITY) • Phase transformations E. Kozeschnik, B. Buchmayr, “Mat. Calc – A simulation tool for multicomponent thermodynamics, diffusion and phase transformation kinetics”, in: ‘Mathematical Modelling of Weld Phenomena 5’, Institute of Materials, London, Book 734, 2001; 349. IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

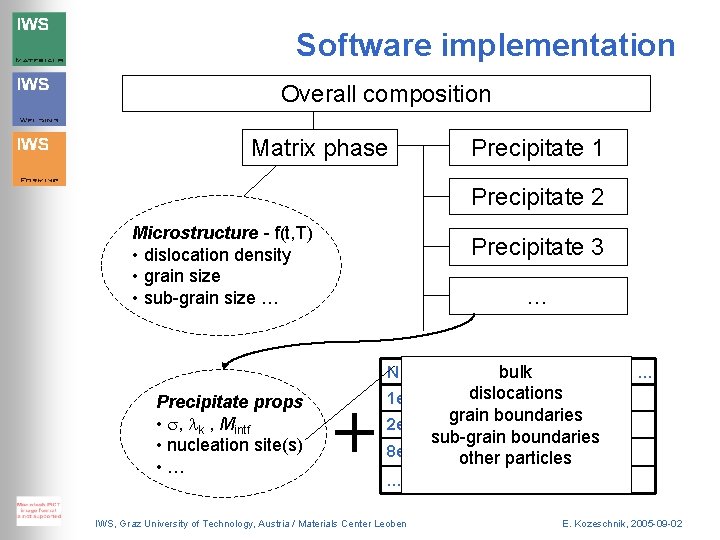
Software implementation Overall composition Matrix phase Precipitate 1 Precipitate 2 Microstructure - f(t, T) • dislocation density • grain size • sub-grain size … Precipitate 3 … N Precipitate props • s, lk , Mintf • nucleation site(s) • … + R XCbulk XCr 1 e 12 4 e-9 dislocations 0. 25 0. 36 XFe … 0. 12 grain boundaries 0. 25 0. 38 0. 11 sub-grain boundaries 8 e 13 6 e-9 0. 25 0. 39 0. 09 other particles 2 e 13 5 e-9 … IWS, Graz University of Technology, Austria / Materials Center Leoben … … E. Kozeschnik, 2005 -09 -02

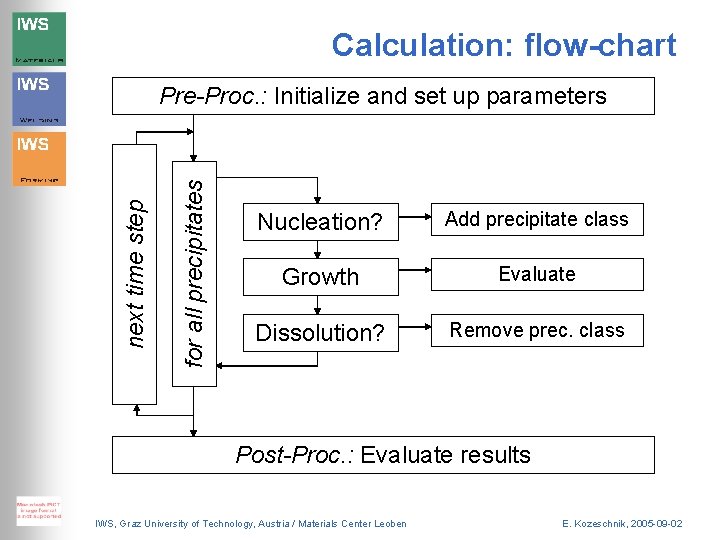
Calculation: flow-chart for all precipitates next time step Pre-Proc. : Initialize and set up parameters Nucleation? Add precipitate class Growth Evaluate Dissolution? Remove prec. class Post-Proc. : Evaluate results IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Example I Nucleation – Growth Coarsening

Live demo. . . • Cementite precipitation in Fe-0. 1%C • 100 precipitate classes • Automatic interfacial energy Start Mat. Calc. . . IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Example II TTP-diagram

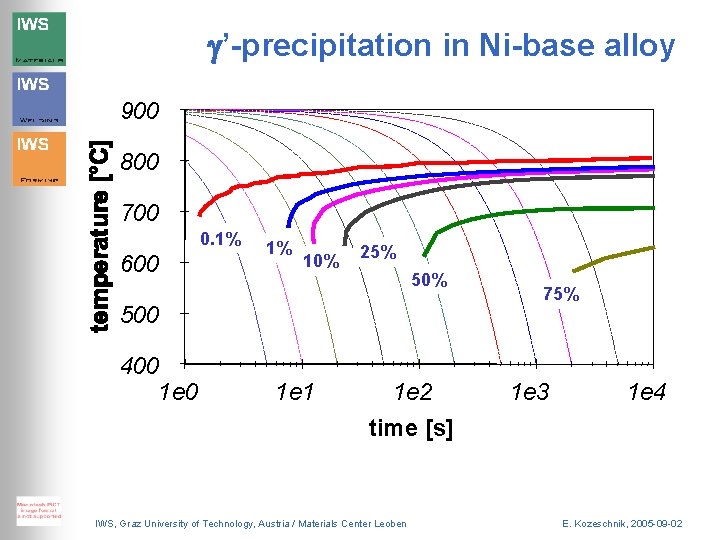
g’-precipitation in Ni-base alloy • • Ni-13 at%Al 200 classes s=17 m. J/m 2 Cooling rates: 0, 01 – 1000 °/s IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

g’-precipitation in Ni-base alloy 900 800 700 0. 1% 600 1% 10% 25% 500 400 1 e 1 1 e 2 time [s] IWS, Graz University of Technology, Austria / Materials Center Leoben 75% 1 e 3 1 e 4 E. Kozeschnik, 2005 -09 -02

Example III Complex systems. . .

A Comprehensive Treatment of Precipitation Kinetics in Complex Materials B. Sonderegger 1, 6, M. Bischof 2, E. Kozeschnik 1 H. Leitner 2, H. Clemens 2, J. Svoboda 4, F. D. Fischer 3, 5 1: Institute for Materials Science, Welding and Forming, Graz, University of Technology, Austria 2: Dept. of Physical Metallurgy and Materials Testing, Montanuniversität Leoben, Austria 3: Institute of Mechanics, Montanuniversität Leoben, Austria 4: Institute of Physics of Materials, Academy of Sciences of the Czech Republic, Brno, Czech Republic 5: Erich Schmid Institute of Materials Science, Austrian Academy of Sciences, Leoben, Austria 6: Materials Center Leoben, Austria Presentation given at „Solid-solid Phase Transformations in Inorganic Materials“, Phoenix, AZ, USA, 2005 IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Outline Introduction Experimental Numerical Results ! Conclusion IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Complex material • Experimental Results Outline • Numerical Simulations Improved Understanding of Precipitation Kinetics IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Introduction Precipitation Hardening in Steels Carbides, Nitrides Intermetallic Phases (e. g maraging steels) Testmelt IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

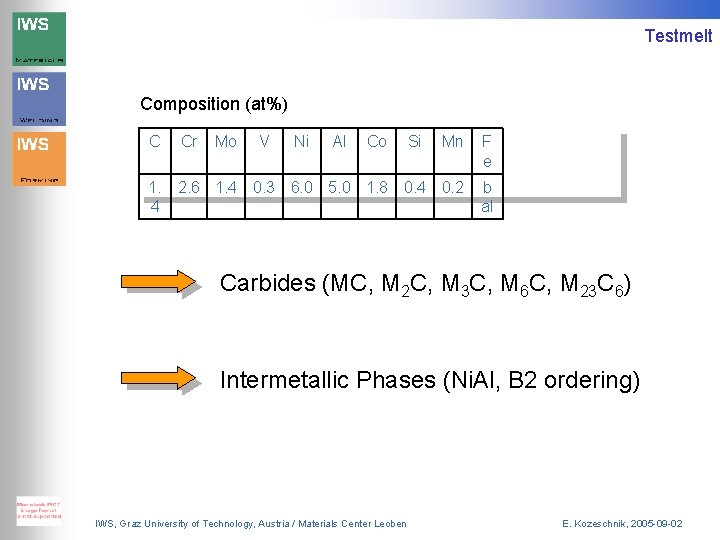
Testmelt Composition (at%) C Cr Mo V Ni Al Co Si Mn F e 1. 4 2. 6 1. 4 0. 3 6. 0 5. 0 1. 8 0. 4 0. 2 b al Carbides (MC, M 2 C, M 3 C, M 6 C, M 23 C 6) Intermetallic Phases (Ni. Al, B 2 ordering) IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

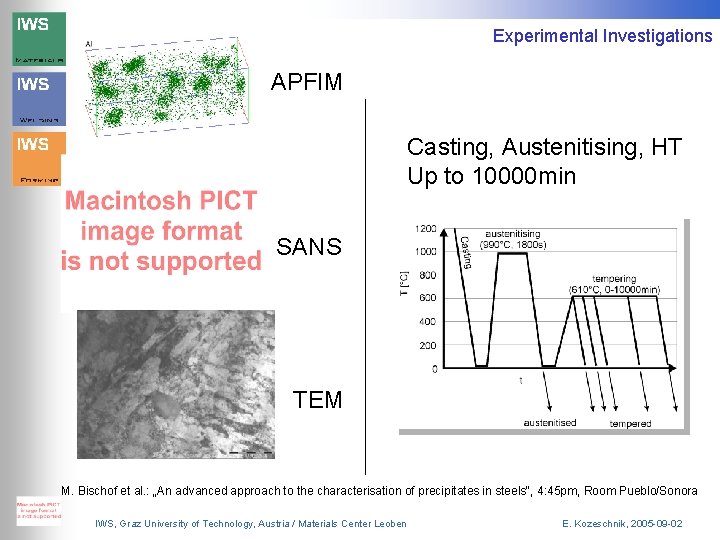
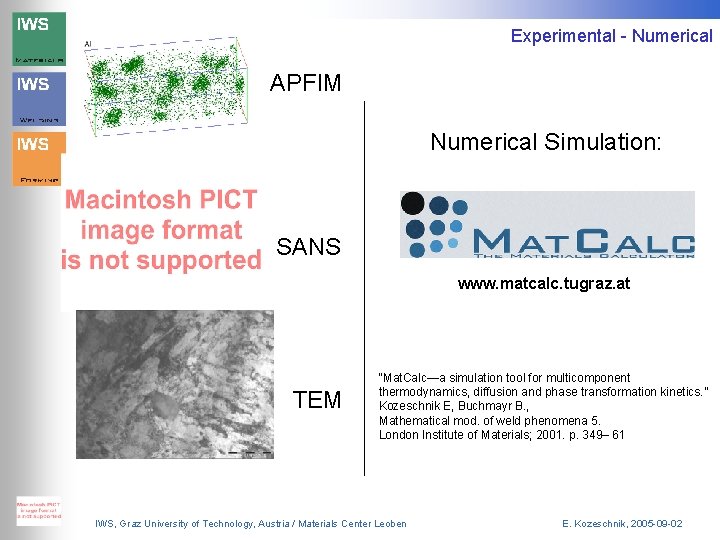
Experimental Investigations APFIM Casting, Austenitising, HT Up to 10000 min SANS TEM M. Bischof et al. : „An advanced approach to the characterisation of precipitates in steels“, 4: 45 pm, Room Pueblo/Sonora IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Experimental - Numerical APFIM Numerical Simulation: SANS www. matcalc. tugraz. at TEM “Mat. Calc—a simulation tool for multicomponent thermodynamics, diffusion and phase transformation kinetics. ” Kozeschnik E, Buchmayr B. , Mathematical mod. of weld phenomena 5. London Institute of Materials; 2001. p. 349– 61 IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

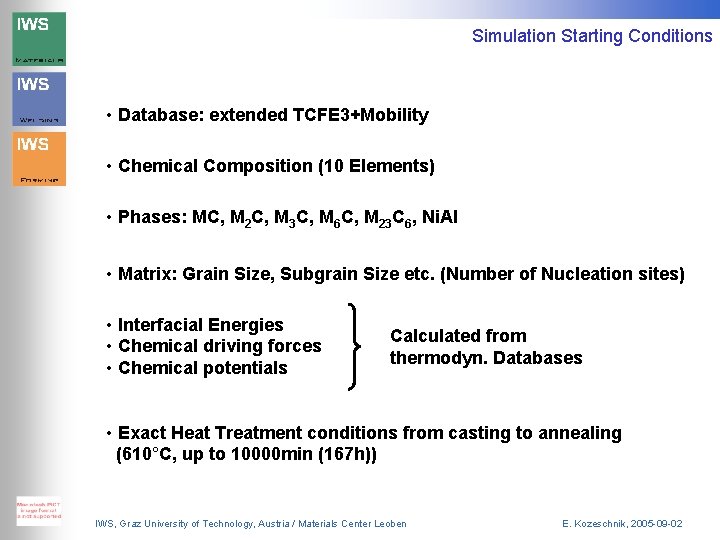
Simulation Starting Conditions • Database: extended TCFE 3+Mobility • Chemical Composition (10 Elements) • Phases: MC, M 2 C, M 3 C, M 6 C, M 23 C 6, Ni. Al • Matrix: Grain Size, Subgrain Size etc. (Number of Nucleation sites) • Interfacial Energies • Chemical driving forces • Chemical potentials Calculated from thermodyn. Databases • Exact Heat Treatment conditions from casting to annealing (610°C, up to 10000 min (167 h)) IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

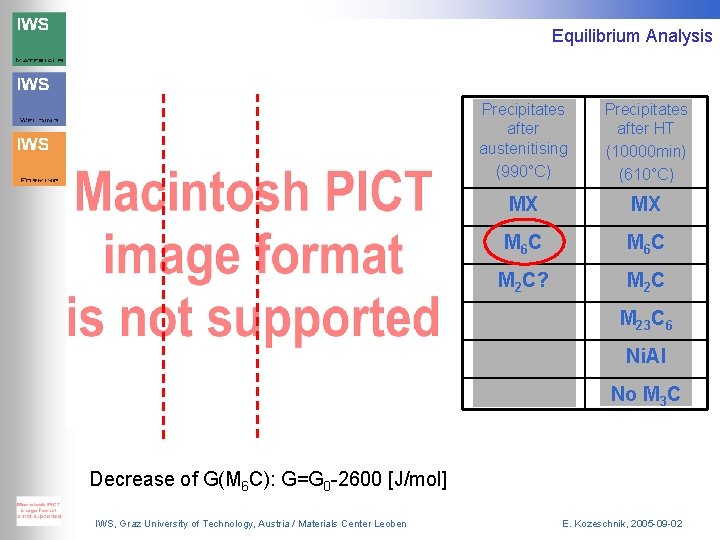
Equilibrium Analysis Precipitates after austenitising (990°C) Precipitates after HT (10000 min) (610°C) MX MX M 6 C M 2 C? M 2 C M 23 C 6 Ni. Al No M 3 C Decrease of G(M 6 C): G=G 0 -2600 [J/mol] IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02
![Calculation with improved database GM 6 CG 0 2600 Jmol M 6 C 1 Calculation with improved database G(M 6 C)=G 0 -2600 [J/mol] M 6 C: 1,](https://slidetodoc.com/presentation_image/49ff1ef4610e64ca98707685ed5e888c/image-27.jpg)
Calculation with improved database G(M 6 C)=G 0 -2600 [J/mol] M 6 C: 1, 5 mol%, d=580 nm MX: 0, 2 mol%, d=60 nm M 2 C: very few primary Improved Database IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

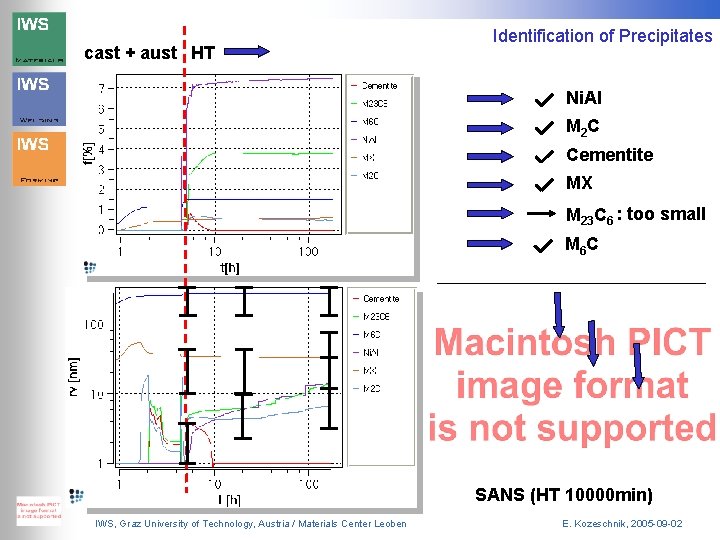
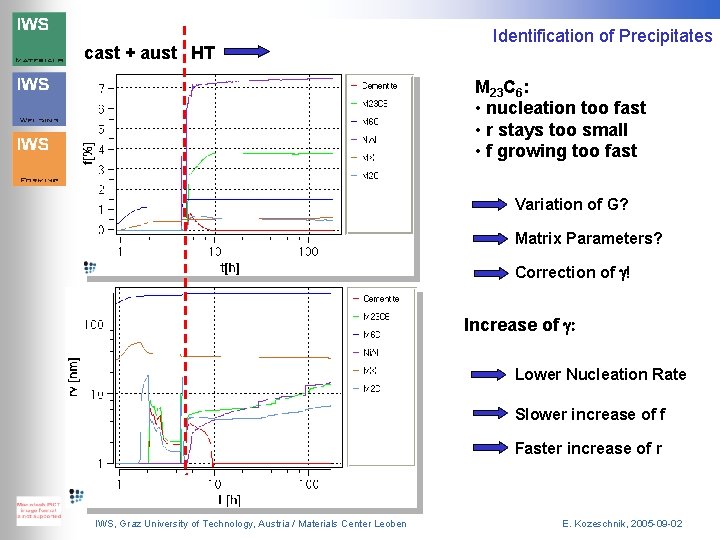
cast + aust HT Identification of Precipitates Ni. Al M 2 C Cementite MX M 23 C 6 : too small M 6 C SANS (HT 10000 min) IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

cast + aust HT Identification of Precipitates M 23 C 6: • nucleation too fast • r stays too small • f growing too fast Variation of G? Matrix Parameters? Correction of g! Increase of g: Lower Nucleation Rate Slower increase of f Faster increase of r IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

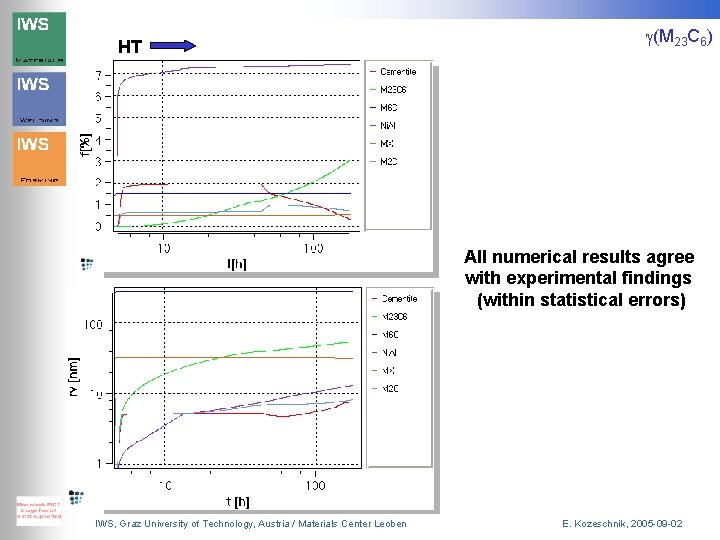
HT g(M 23 C 6) All numerical results agree with experimental findings (within statistical errors) IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

Summary and Conclusions Simulated full heat treatment of a very complex system (10 Elements, 6 phases) Correct Equlibrium Calculations Very good results of kinetic simulation Fit of 2 parameters were sufficient to meet ~ 20 -30 single measurement points Experiments get easier to interpret Simulation results get improved Further development of thermodynamic databases IWS, Graz University of Technology, Austria / Materials Center Leoben E. Kozeschnik, 2005 -09 -02

http: //matcalc. tugraz. at