MMWave Rotational Spectrum of Methyl Nitrate Jessica Thomas



- Slides: 15

MM-Wave Rotational Spectrum of Methyl Nitrate Jessica Thomas, Ivan Medvedev, Department of Physics, Wright State University David Dolson Department of Chemistry, Wright State University

OUR INTEREST IN METHYL NITRATE • Chemical related to metabolism • With diabetes this chemical has been known to be on the order of parts per trillion. • Also, it is known to be expressed in breath in children with type 1 diabetes. • Likely, oxidative stress on individuals during hyperglycemia in type 1 diabetics causes methyl nitrate production. • Could be an indicator used for air quality monitoring • Possesses explosive qualities • Could be a molecule of interest in interstellar medium http: //commons. wikimedia. org/wiki/File: Methyl_nitrate_3 D_ball. png

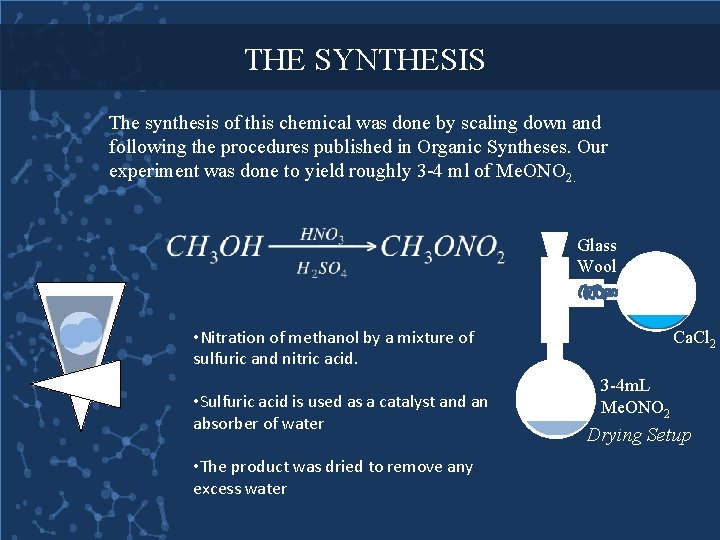
THE SYNTHESIS The synthesis of this chemical was done by scaling down and following the procedures published in Organic Syntheses. Our experiment was done to yield roughly 3 -4 ml of Me. ONO 2. Glass Wool • Nitration of methanol by a mixture of sulfuric and nitric acid. • Sulfuric acid is used as a catalyst and an absorber of water • The product was dried to remove any excess water Ca. Cl 2 3 -4 m. L Me. ONO 2 Drying Setup

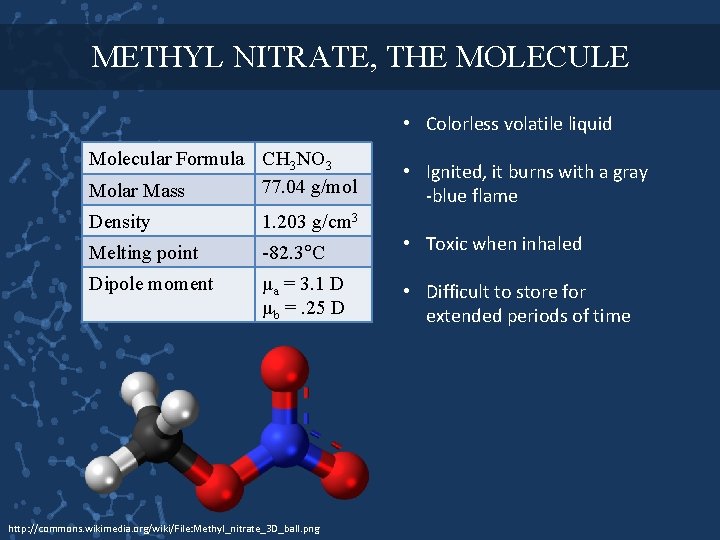
METHYL NITRATE, THE MOLECULE • Colorless volatile liquid Molecular Formula CH 3 NO 3 77. 04 g/mol Molar Mass • Ignited, it burns with a gray -blue flame Density 1. 203 g/cm 3 Melting point -82. 3 C • Toxic when inhaled Dipole moment μa = 3. 1 D μb =. 25 D • Difficult to store for extended periods of time http: //commons. wikimedia. org/wiki/File: Methyl_nitrate_3 D_ball. png

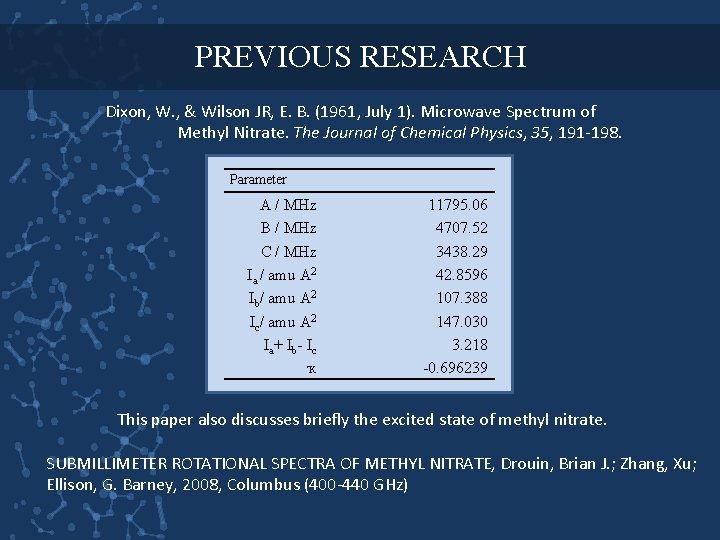
PREVIOUS RESEARCH Dixon, W. , & Wilson JR, E. B. (1961, July 1). Microwave Spectrum of Methyl Nitrate. The Journal of Chemical Physics, 35, 191 -198. Parameter A / MHz B / MHz C / MHz Ia / amu A 2 Ib/ amu A 2 Ic/ amu A 2 Ia+ Ib- Ic ҡ 11795. 06 4707. 52 3438. 29 42. 8596 107. 388 147. 030 3. 218 -0. 696239 This paper also discusses briefly the excited state of methyl nitrate. SUBMILLIMETER ROTATIONAL SPECTRA OF METHYL NITRATE, Drouin, Brian J. ; Zhang, Xu; Ellison, G. Barney, 2008, Columbus (400 -440 GHz)

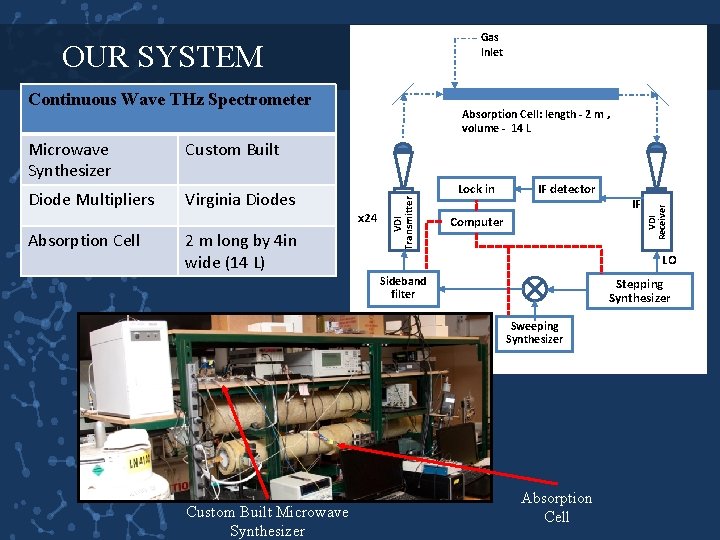
Gas Inlet OUR SYSTEM Continuous Wave THz Spectrometer Diode Multipliers Virginia Diodes Absorption Cell 2 m long by 4 in wide (14 L) x 24 Lock in IF detector Computer LO Sideband filter Stepping Synthesizer Sweeping Synthesizer Custom Built Microwave Synthesizer IF VDI Receiver Custom Built VDI Transmitter Microwave Synthesizer Absorption Cell: length - 2 m , volume - 14 L Absorption Cell

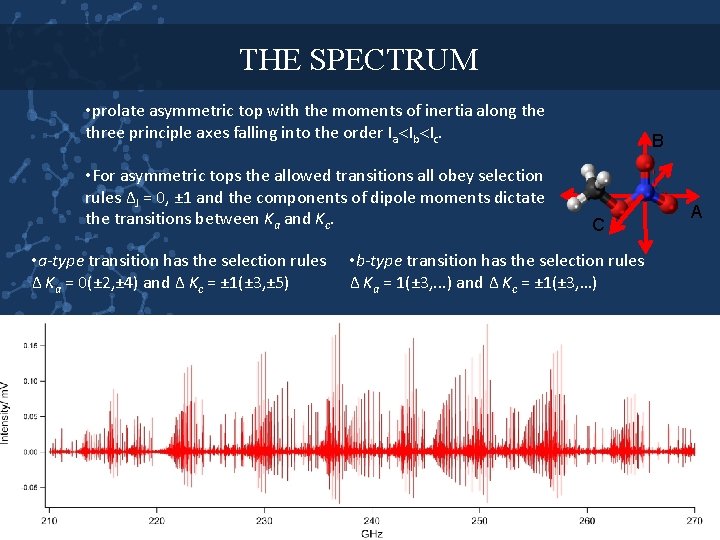
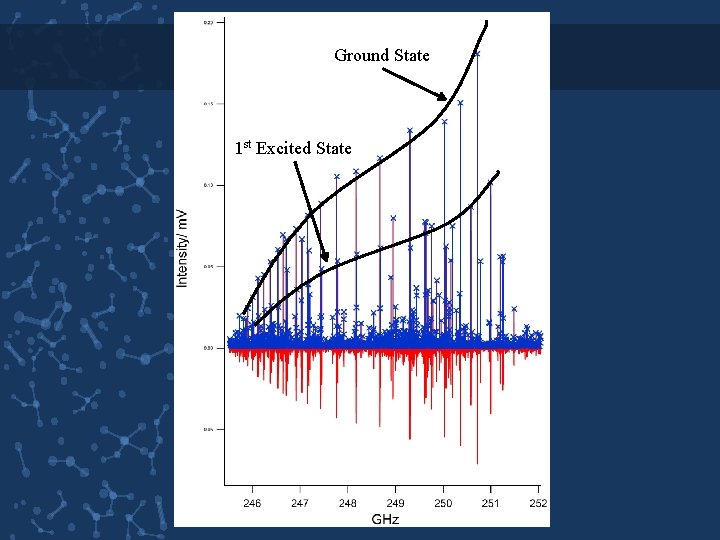
THE SPECTRUM • prolate asymmetric top with the moments of inertia along the three principle axes falling into the order Ia Ib Ic. • For asymmetric tops the allowed transitions all obey selection rules ∆J = 0, ± 1 and the components of dipole moments dictate the transitions between Ka and Kc. • a-type transition has the selection rules ∆ Ka = 0(± 2, ± 4) and ∆ Kc = ± 1(± 3, ± 5) B C • b-type transition has the selection rules ∆ Ka = 1(± 3, . . . ) and ∆ Kc = ± 1(± 3, …) A


Ground State 1 st Excited State

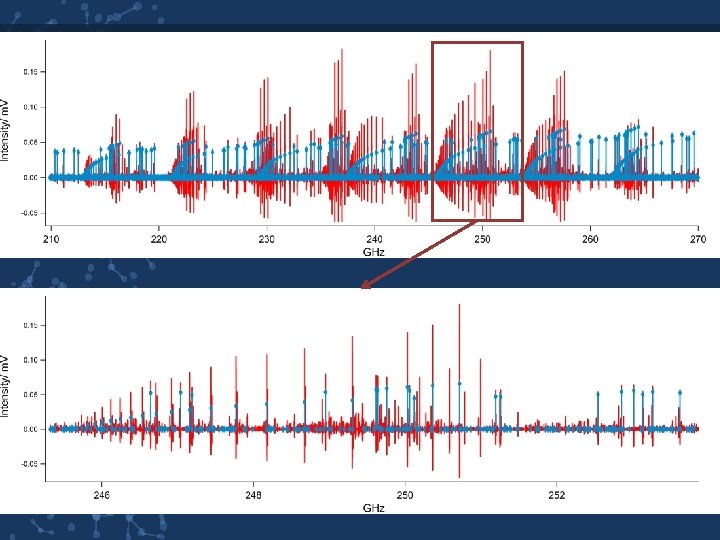
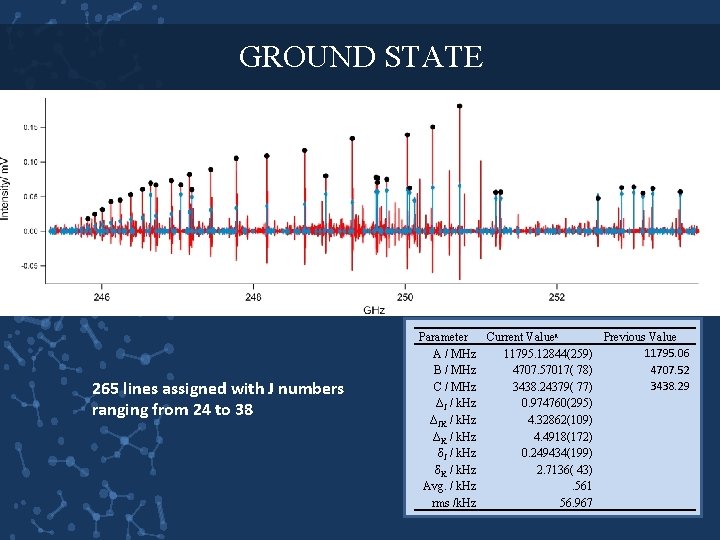
GROUND STATE 265 lines assigned with J numbers ranging from 24 to 38 Parameter Current Valuea Previous Value 11795. 06 A / MHz 11795. 12844(259) 4707. 52 B / MHz 4707. 57017( 78) 3438. 29 C / MHz 3438. 24379( 77) ΔJ / k. Hz 0. 974760(295) ΔJK / k. Hz 4. 32862(109) ΔK / k. Hz 4. 4918(172) δJ / k. Hz 0. 249434(199) δK / k. Hz 2. 7136( 43) Avg. / k. Hz. 561 rms /k. Hz 56. 967

NUMERICS Parameter Old Values Current Value A / MHz 11795. 06 11795. 12844(259) B / MHz 4707. 52 4707. 57017( 78)) C / MHz 3438. 29 3438. 24379( 77) Ia / amu A 2 42. 8596 42. 85930438 Ib/ amu A 2 107. 388 107. 386822 Ic/ amu A 2 147. 030 147. 0317496 Ia+ Ib- Ic 3. 218 3. 214376799 ҡ -0. 696239 -0. 696220199

1 ST EXCITED STATE

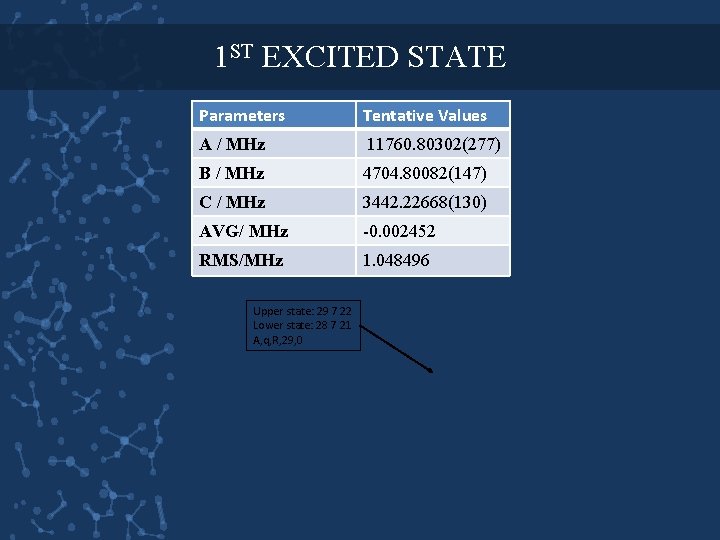
1 ST EXCITED STATE Perturbed lines from likely a combination of the v=1 methyl torsion and the v=1 NO 2 torsion. Upper state: 30 6 25 Lower state: 29 6 24 a, q, R, 30, -1 Upper state: 31 5 27 Lower state: 30 5 26 a, q, R, 31, -1 Upper state: 28 8 20 Lower state: 27 8 19 a, q, R, 28, 0 Upper state: 31 4 27 Lower state: 30 4 26 a, q, R, 31, 0

1 ST EXCITED STATE Parameters Tentative Values A / MHz 11760. 80302(277) B / MHz 4704. 80082(147) C / MHz 3442. 22668(130) AVG/ MHz -0. 002452 RMS/MHz 1. 048496 Upper state: 29 7 22 Lower state: 28 7 21 A, q, R, 29, 0

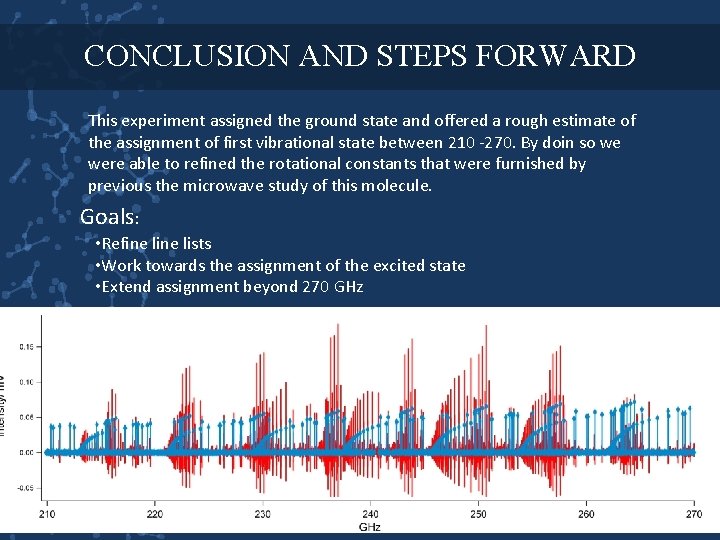
CONCLUSION AND STEPS FORWARD This experiment assigned the ground state and offered a rough estimate of the assignment of first vibrational state between 210 -270. By doin so we were able to refined the rotational constants that were furnished by previous the microwave study of this molecule. Goals: • Refine lists • Work towards the assignment of the excited state • Extend assignment beyond 270 GHz
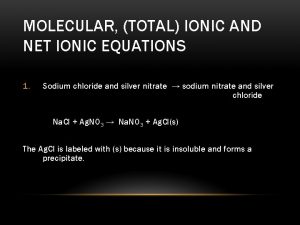
 Sodium nitrate and silver nitrate
Sodium nitrate and silver nitrate Example of a chemical change
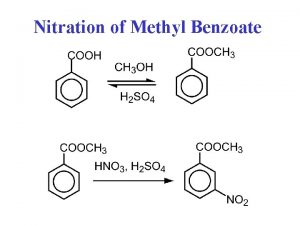
Example of a chemical change Nitration of methyl benzoate ortho meta para
Nitration of methyl benzoate ortho meta para Rotational equilibrium example problems
Rotational equilibrium example problems Rotational equilibrium and rotational dynamics
Rotational equilibrium and rotational dynamics Chromium orbital configuration
Chromium orbital configuration Absortpion
Absortpion Thomas hobbes political spectrum
Thomas hobbes political spectrum Thomas mocker and thomas stewart
Thomas mocker and thomas stewart Methyl group ortho para directing
Methyl group ortho para directing Butanol
Butanol Isopropyl methyl ether
Isopropyl methyl ether Methyl cyanide with grignard reagent
Methyl cyanide with grignard reagent 3-methylcyklopentanon
3-methylcyklopentanon Definition of methyl orange
Definition of methyl orange Ncnh- lewis structure
Ncnh- lewis structure