METHYL ORANGE DEFINITION Methyl orange is a p







- Slides: 11

METHYL ORANGE

DEFINITION � Methyl orange is a p. H indicator frequently used in titration because of its clear and distinct color variance at different p. H values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p. H of a mid strength acid, it is usually used in titration for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point

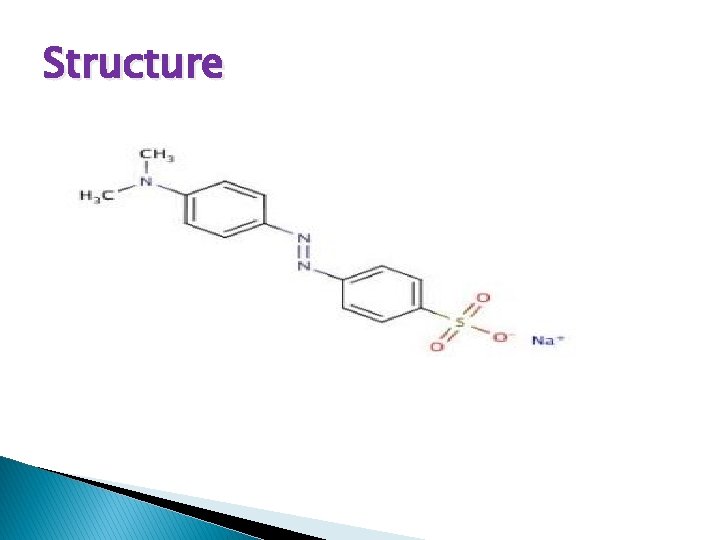
Structure

Indicator

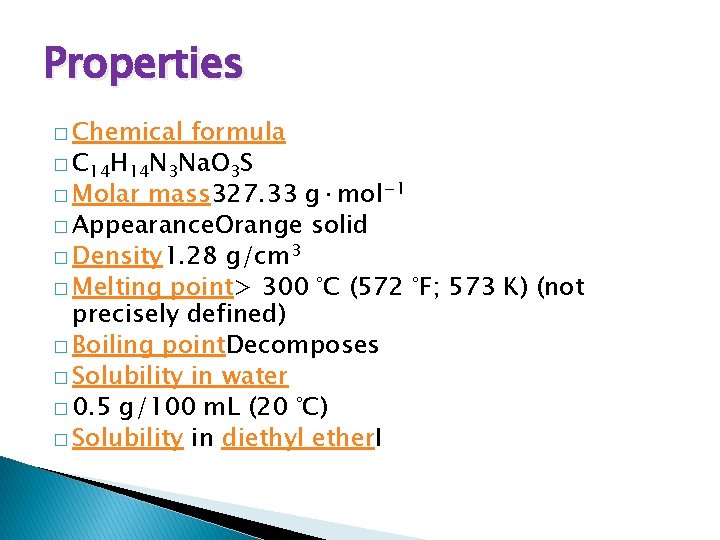
Properties � Chemical formula � C 14 H 14 N 3 Na. O 3 S � Molar mass 327. 33 g·mol− 1 � Appearance. Orange solid � Density 1. 28 g/cm 3 � Melting point> 300 °C (572 °F; 573 K) (not precisely defined) � Boiling point. Decomposes � Solubility in water � 0. 5 g/100 m. L (20 °C) � Solubility in diethyl ether. I

� In a solution becoming less acidic, methyl orange moves from red to orange and finally to yellow with the reverse occurring for a solution increasing in acidity. The entire color change occurs in acidic conditions. � Methyl orange (p. H indicator)below p. H 3. 1 above p. H 4. 43. 1⇌4. 4 In an acid, it is reddish and in alkali, it is yellow. Methyl orange

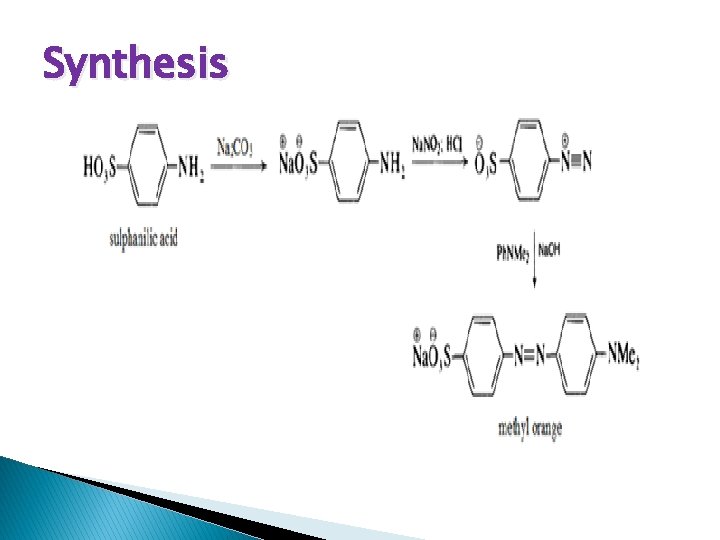
Synthesis

Procedure � 1 mole sulfanilic acid is dissolved in exactly 1 mole dilute sodium hydroxide, treated with 1 mole sodium nitrite, and then 1 mole hydrochloric acid is added in the cold. This solution is treated straight away with 1 mole dimethylaniline in a little hydrochloric acid, and sodium hyroxide is again added. The sodium salt of the methyl orange soon separates out. The precipitation may be rendered more complete by adding common salt.

What does methyl orange indicate? � Methyl orange is a p. H indicator frequently used in titration because of its clear and distinct color variance at different p. H values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p. H of a mid strength acid, it is usually used in titration for acids.

Why is methyl orange used in titration? � Methyl orange is a p. H indicator frequently used in titrations because of its clear and distinct colour change. Because it changes colour at the p. H of a midstrength acid, it is usually used in titrations for acids.

THANK YOU