Rotational Spectrum of the Methyl Salicylate Water Complex

- Slides: 19

Rotational Spectrum of the Methyl Salicylate – Water Complex: The Missing Conformer and the Tunneling Motions Supriya Ghosh, Javix Thomas, Yunjie Xu, and Wolfgang Jäger Department of Chemistry, University of Alberta, Edmonton, Canada

Methyl Salicylate (Oil of Wintergreen) Occurs in leaves of plants (abundant in Sweet Birch and Eastern Teaberry or Wintergreen) Used in ointments to relief minor aches and pains (Ben Gay, Icy Hot). 2

Atmospheric Significance • Methyl salicylate is a biogenic volatile organic compound (VOC) • Forms secondary organic aerosols in both gaseous and aqueous phases • Is a surfactant and enhances activity of cloud condensation nuclei

Chemical Communication Some Plants release volatile chemicals in stress conditions (cold, heat, drought, insects, disease). This triggers defensive response in themselves and in neighbouring plants. Intraspecific signalling Auto-signalling Interspecific signalling Victim of attack http: //www. howplantswork. com/2011/07/16/talking-plants-airborne-chemical-signals-part-2/ Sang-Wook et al. Science 318, 113 (2007); Vladimir et al. Nature 385, 718 (1997). 4

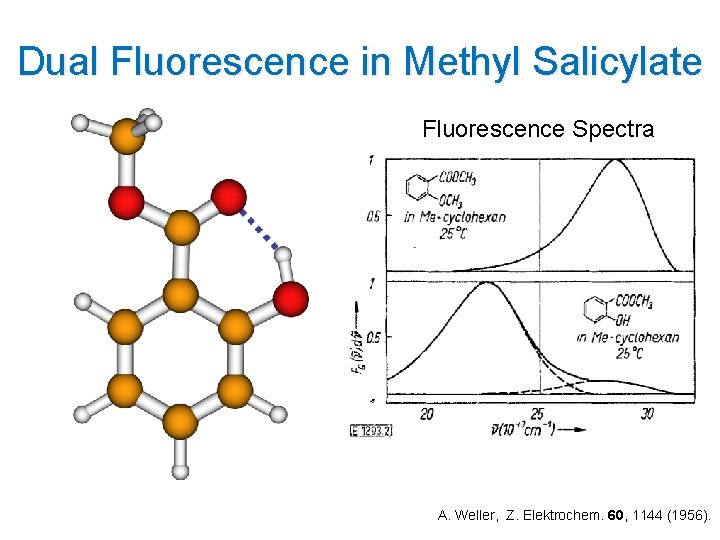
Dual Fluorescence in Methyl Salicylate Fluorescence Spectra A. Weller, Z. Elektrochem. 60, 1144 (1956).

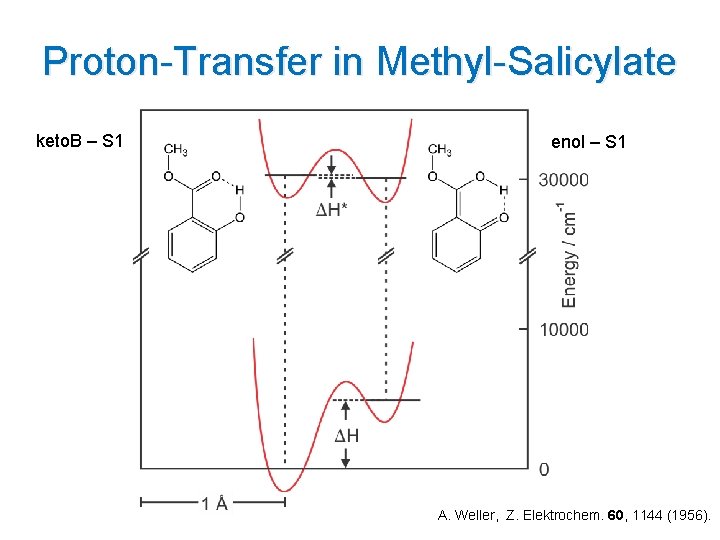
Proton-Transfer in Methyl-Salicylate keto. B – S 1 enol – S 1 A. Weller, Z. Elektrochem. 60, 1144 (1956).

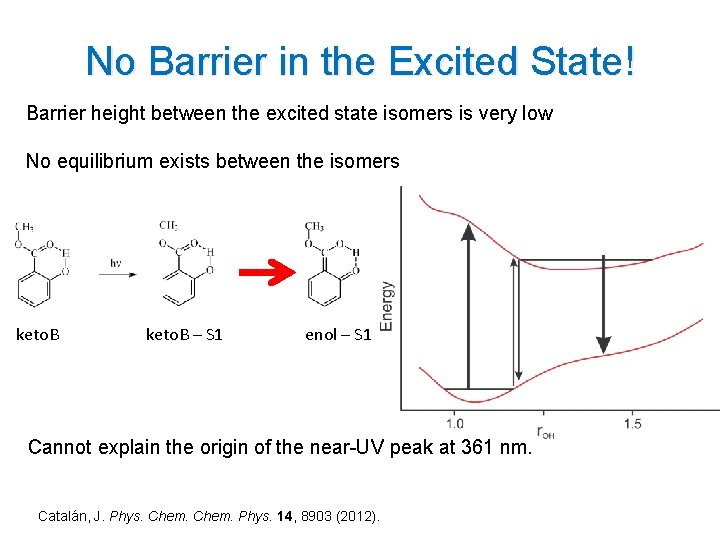
No Barrier in the Excited State! Barrier height between the excited state isomers is very low No equilibrium exists between the isomers keto. B – S 1 enol – S 1 Cannot explain the origin of the near-UV peak at 361 nm. Catalán, J. Phys. Chem. Phys. 14, 8903 (2012). 7

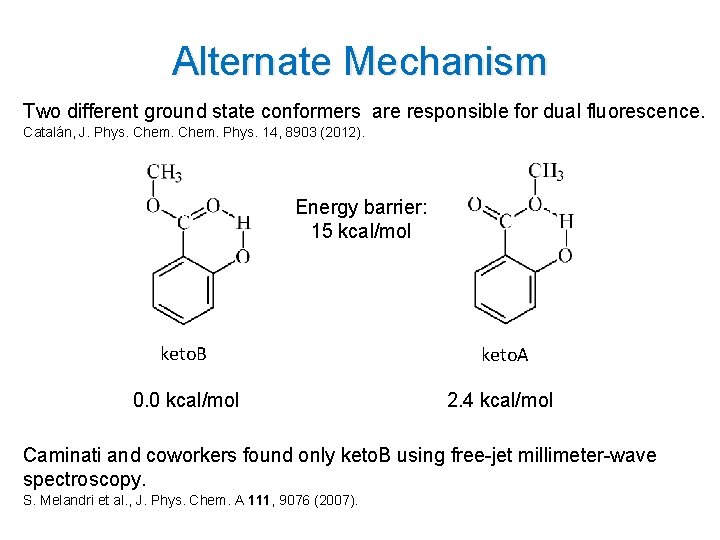
Alternate Mechanism Two different ground state conformers are responsible for dual fluorescence. Catalán, J. Phys. Chem. Phys. 14, 8903 (2012). Energy barrier: 15 kcal/mol keto. B keto. A 0. 0 kcal/mol 2. 4 kcal/mol Caminati and coworkers found only keto. B using free-jet millimeter-wave spectroscopy. S. Melandri et al. , J. Phys. Chem. A 111, 9076 (2007). 8

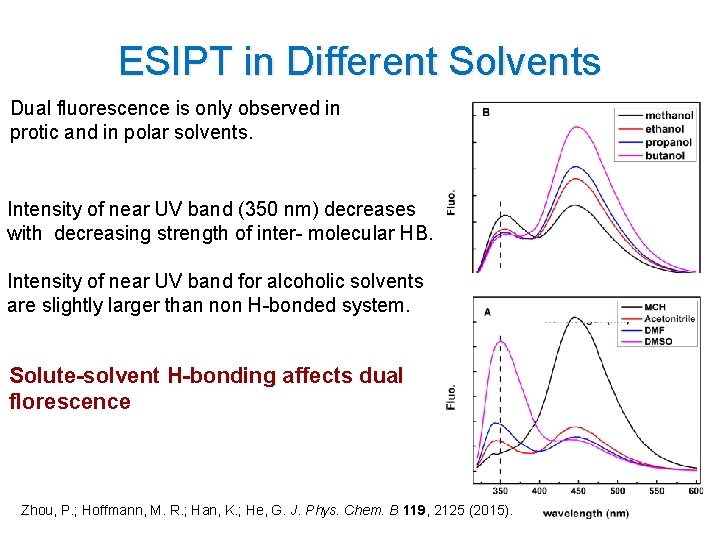
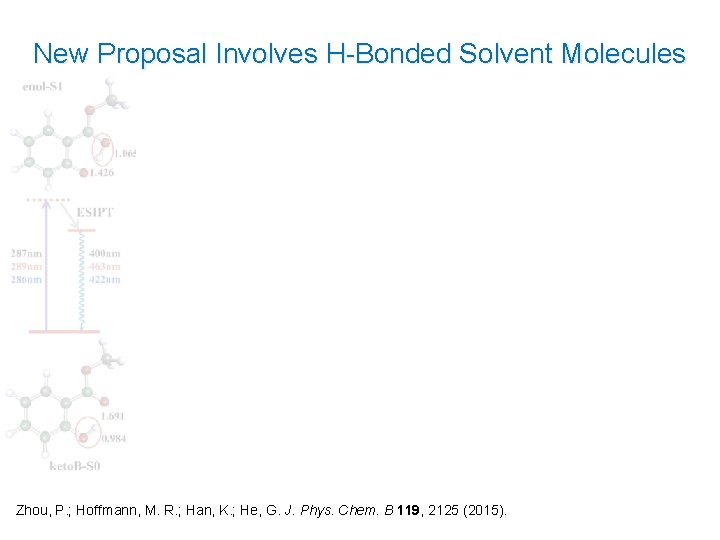
ESIPT in Different Solvents Dual fluorescence is only observed in protic and in polar solvents. Intensity of near UV band (350 nm) decreases with decreasing strength of inter- molecular HB. Intensity of near UV band for alcoholic solvents are slightly larger than non H-bonded system. Solute-solvent H-bonding affects dual florescence Zhou, P. ; Hoffmann, M. R. ; Han, K. ; He, G. J. Phys. Chem. B 119, 2125 (2015). 9

New Proposal Involves H-Bonded Solvent Molecules enol-meth. A-S 1 -> blue band keto. B-meth. B-S 1 -> near UV band Responsible for dual fluorescence Zhou, P. ; Hoffmann, M. R. ; Han, K. ; He, G. J. Phys. Chem. B 119, 2125 (2015). 10

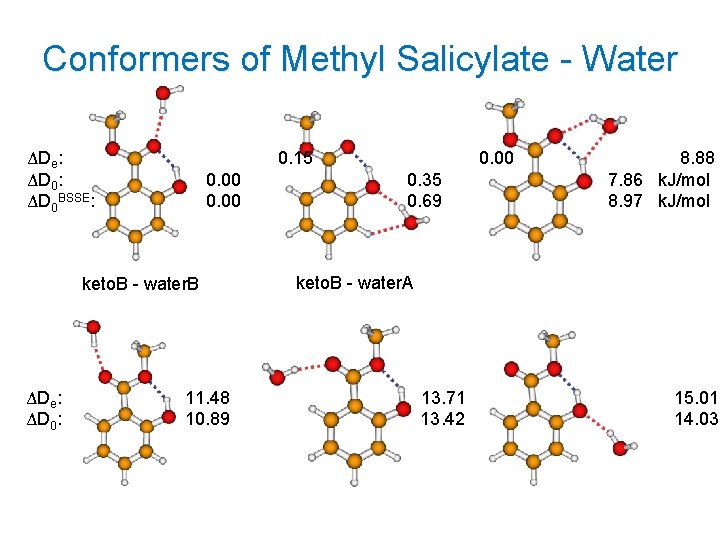
Conformers of Methyl Salicylate - Water ΔDe: ΔD 0 BSSE: 0. 15 0. 00 keto. B - water. B ΔDe: ΔD 0: 11. 48 10. 89 0. 00 0. 35 0. 69 8. 88 7. 86 k. J/mol 8. 97 k. J/mol keto. B - water. A 13. 71 13. 42 15. 01 14. 03

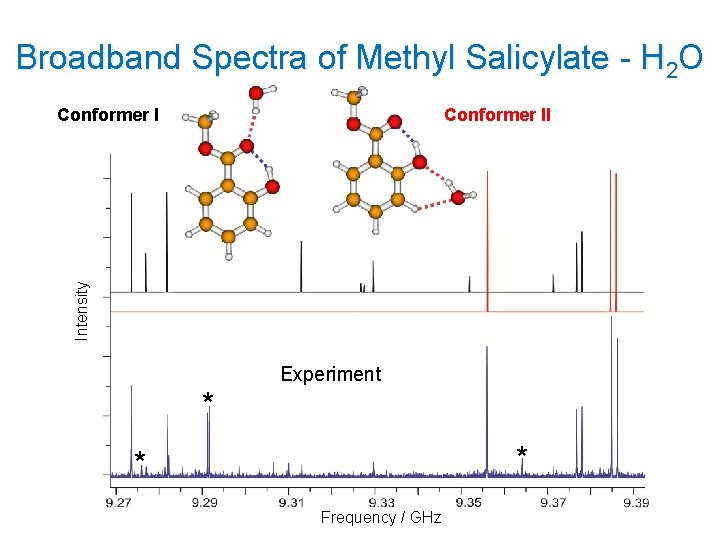
Broadband Spectra of Methyl Salicylate - H 2 O Conformer II Intensity Conformer I Experiment * * * Frequency / GHz 12

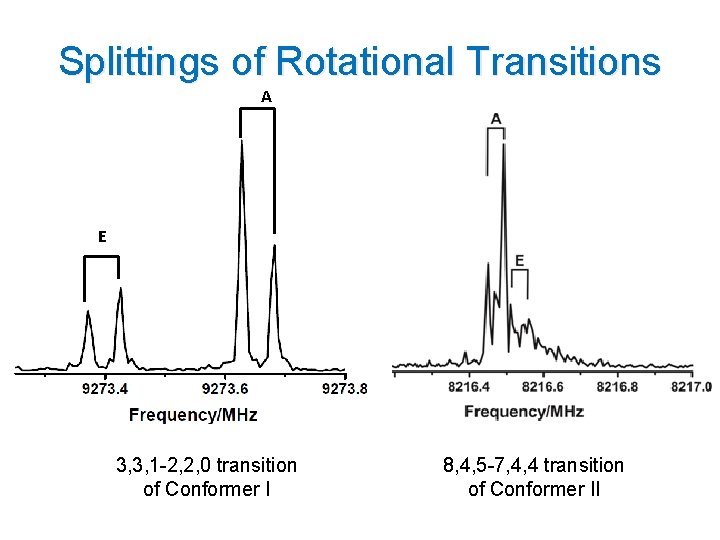
Splittings of Rotational Transitions A E 3, 3, 1 -2, 2, 0 transition of Conformer I 8, 4, 5 -7, 4, 4 transition of Conformer II

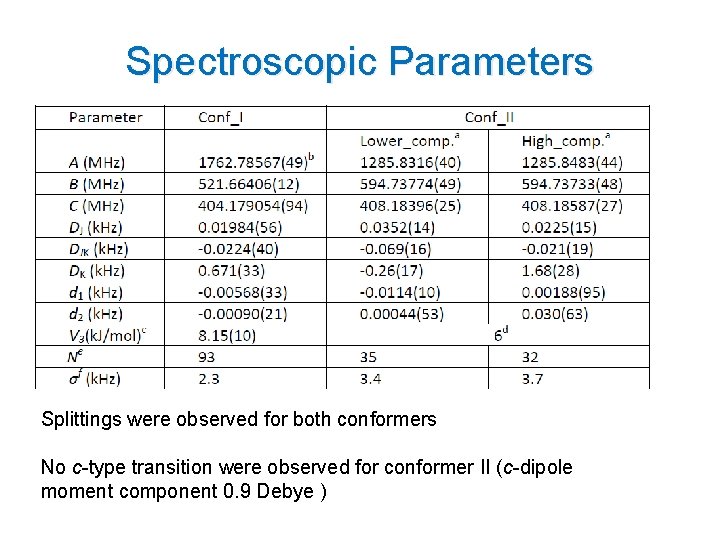
Spectroscopic Parameters Splittings were observed for both conformers No c-type transition were observed for conformer II (c-dipole moment component 0. 9 Debye ) 14

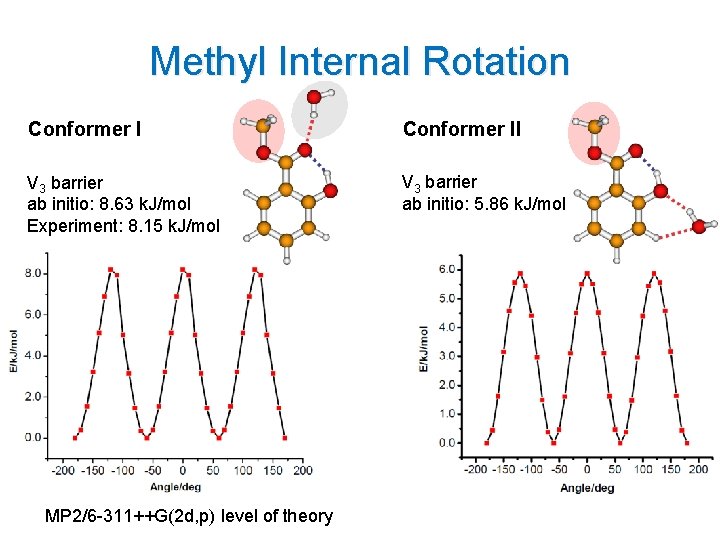
Methyl Internal Rotation Conformer II V 3 barrier ab initio: 8. 63 k. J/mol Experiment: 8. 15 k. J/mol V 3 barrier ab initio: 5. 86 k. J/mol MP 2/6 -311++G(2 d, p) level of theory 15

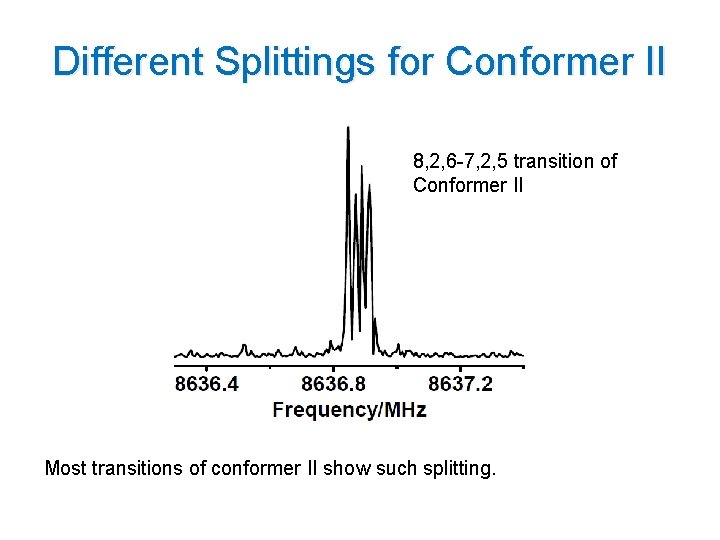
Different Splittings for Conformer II 8, 2, 6 -7, 2, 5 transition of Conformer II Most transitions of conformer II show such splitting. 16

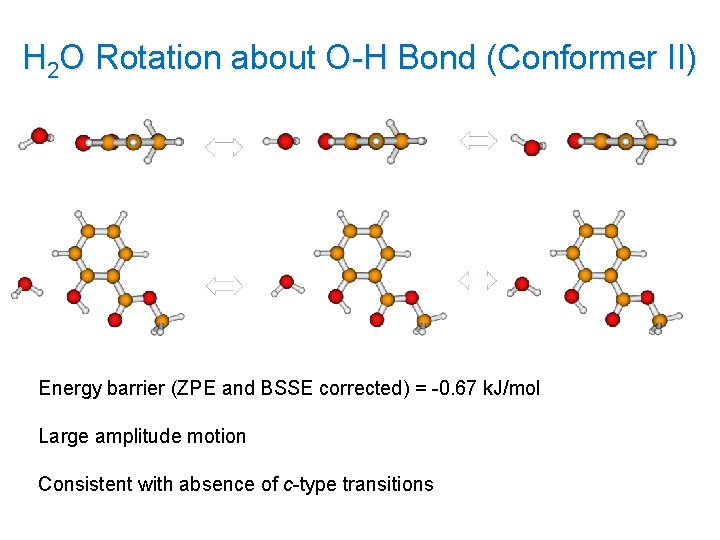
H 2 O Rotation about O-H Bond (Conformer II) Energy barrier (ZPE and BSSE corrected) = -0. 67 k. J/mol Large amplitude motion Consistent with absence of c-type transitions 17

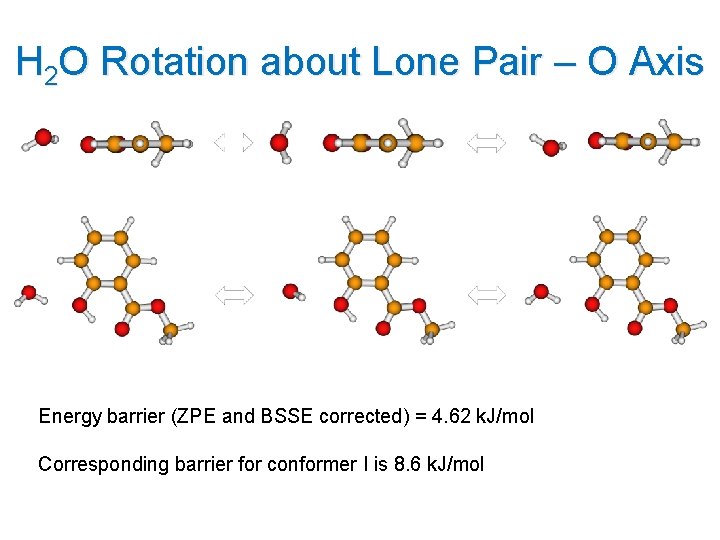
H 2 O Rotation about Lone Pair – O Axis Energy barrier (ZPE and BSSE corrected) = 4. 62 k. J/mol Corresponding barrier for conformer I is 8. 6 k. J/mol 18

Summary Two conformers of methyl salicylate – water were identified. This supports the new proposal for the dual fluorescence signature of methyl salicylate in protic solvents. The search for the missing ground state conformer of methyl salicylate was not successful.
 Salicylic acid disinfectant
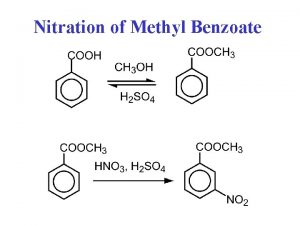
Salicylic acid disinfectant Nitrating methyl benzoate
Nitrating methyl benzoate Fischer esterification methyl salicylate
Fischer esterification methyl salicylate Methyl salicylate classification
Methyl salicylate classification Methyl salicylate classification
Methyl salicylate classification Water and water and water water
Water and water and water water Torque free body diagram
Torque free body diagram Rotational equilibrium and rotational dynamics
Rotational equilibrium and rotational dynamics Treatment of salicylate toxicity
Treatment of salicylate toxicity Julien froustey
Julien froustey D orbital shape
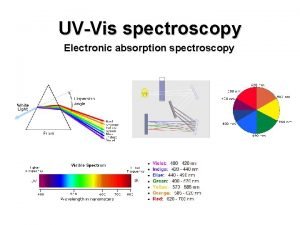
D orbital shape Absortpion
Absortpion Simple compound complex and compound-complex sentences quiz
Simple compound complex and compound-complex sentences quiz Ghon complex and ranke complex
Ghon complex and ranke complex Simple, compound complex rules
Simple, compound complex rules Freud complexes
Freud complexes Carly's therapist asks her to simply
Carly's therapist asks her to simply Thematic apperception
Thematic apperception Deliuded
Deliuded Tert-butyl ir spectrum
Tert-butyl ir spectrum