Experiment 9 Preparation of Methyl Salicyclate H 2





- Slides: 15

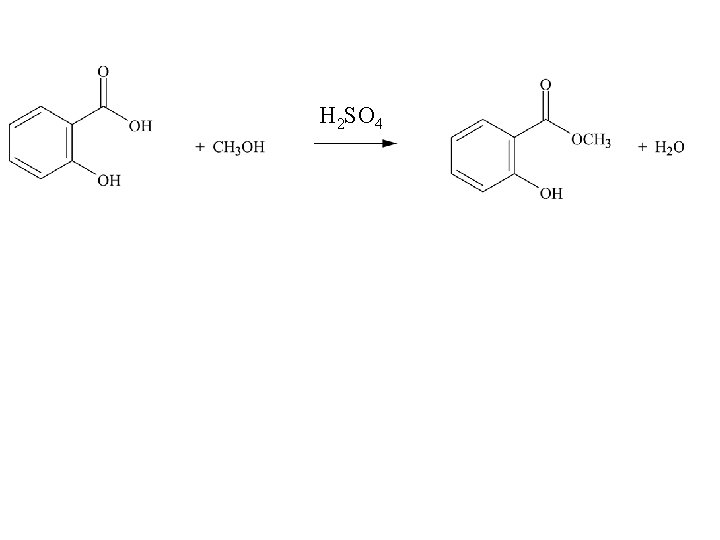
Experiment 9: Preparation of Methyl Salicyclate

H 2 SO 4

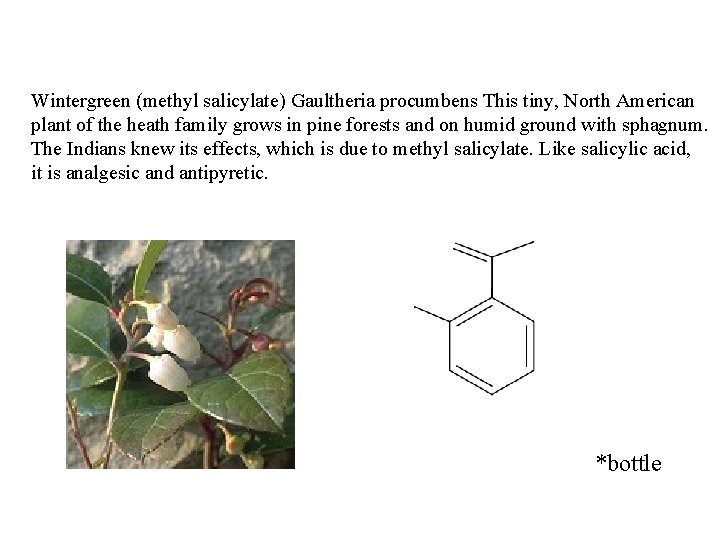
Wintergreen (methyl salicylate) Gaultheria procumbens This tiny, North American plant of the heath family grows in pine forests and on humid ground with sphagnum. The Indians knew its effects, which is due to methyl salicylate. Like salicylic acid, it is analgesic and antipyretic. *bottle

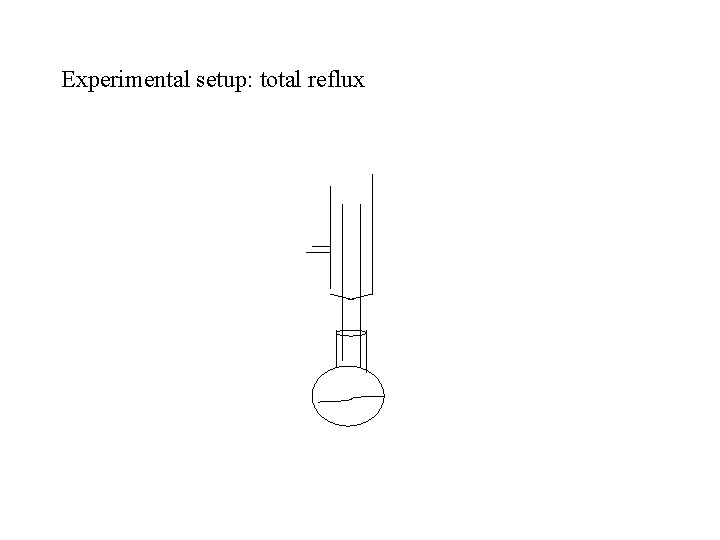
Experimental setup: total reflux


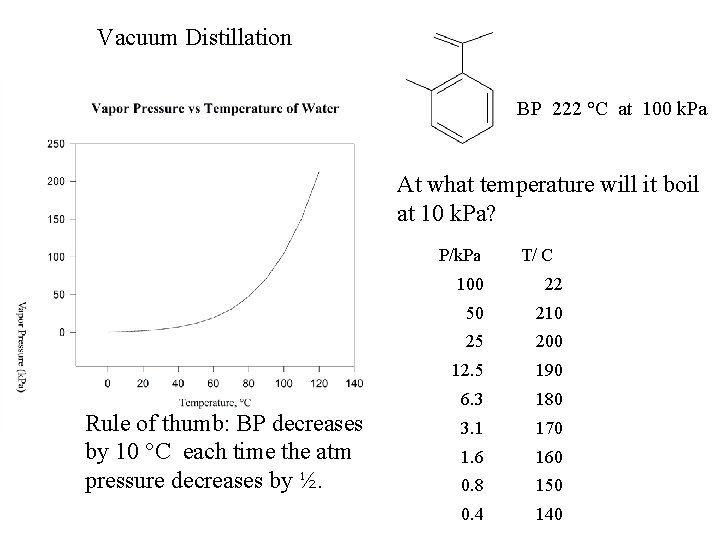
Vacuum Distillation BP 222 °C at 100 k. Pa At what temperature will it boil at 10 k. Pa? P/k. Pa Rule of thumb: BP decreases by 10 °C each time the atm pressure decreases by ½. T/ C 100 22 50 210 25 200 12. 5 190 6. 3 180 3. 1 170 1. 6 160 0. 8 150 0. 4 140

How do I go about figuring out what my unknown is? 1. From your solubility measurements, you should be able to classify your unknowns according to the solubility classes discussed. 2. Look up some reference IR spectra in your manual that have the possible functional groups present in your unknown. 3. Look up some possible classification tests you can run to confirm their presence. 4. Complete and hand in your preliminary report if you haven’t done so. 5. Select a possible derivative based on the properties of the derivative. For example, choose a derivative that will allow you to distinguish between other possible compounds with melting points (± 5 C) or boiling points (± 10 C) within the value of your unknown

Classification Tests Classification test should only be run to confirm your suspicions regarding the presence of a functional group suggested by your solubility tests or IR. Always run a blank and a known at the same time you run your unknown so that you know what a positive test looks like and that you performed the classification test properly. Derivatives A derivative should be chosen whose melting temperature will allow you to differentiate between possible compounds. If you have a carboxylic acid or an amine, a titration can be substituted for a derivative providing that the molecular weights of other possible compounds in your possibility list can be excluded on the basis of your titration results.

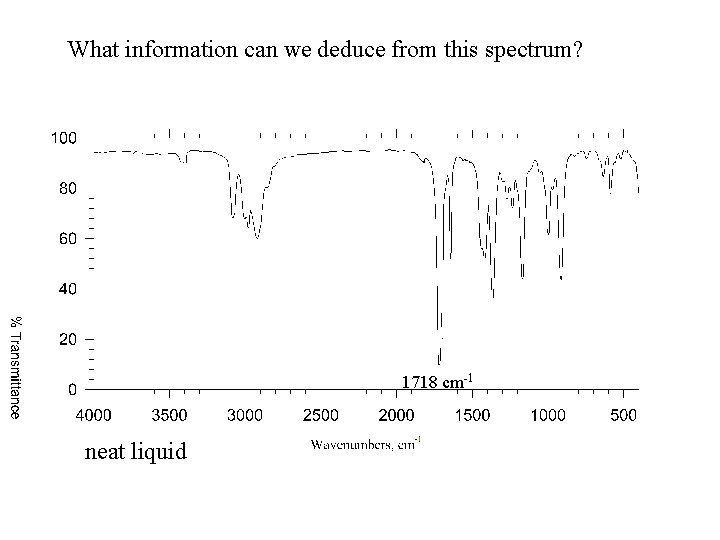
What information can we deduce from this spectrum? 1718 cm-1 neat liquid

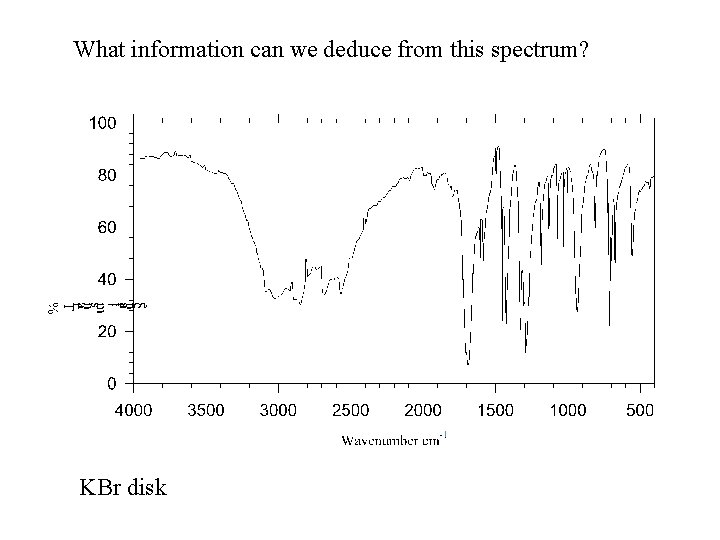
What information can we deduce from this spectrum? KBr disk

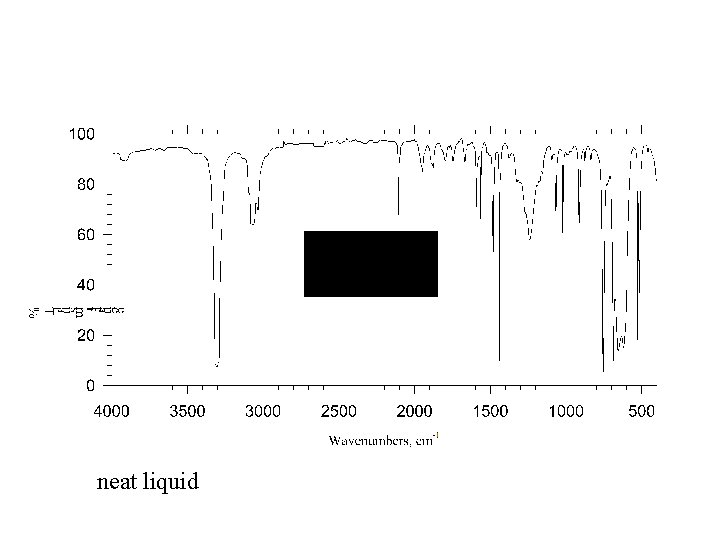
neat liquid

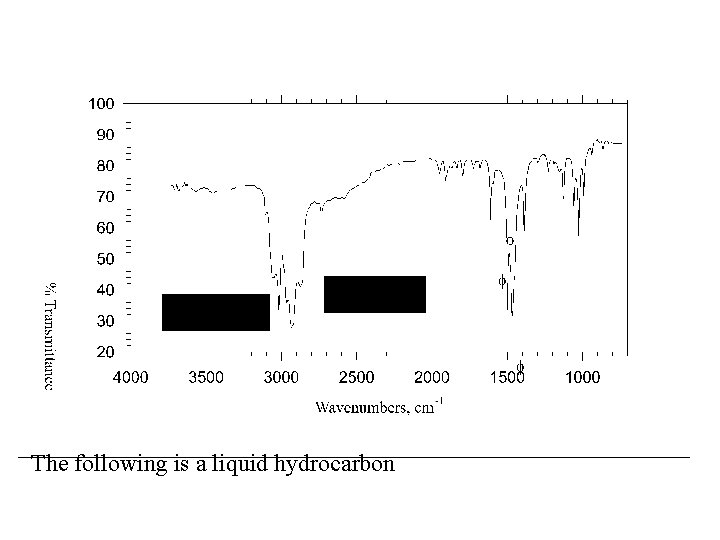
The following is a liquid hydrocarbon

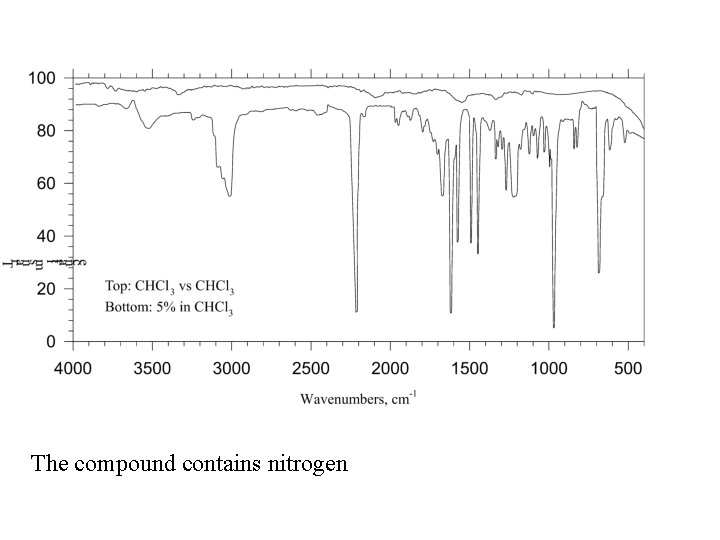
The compound contains nitrogen

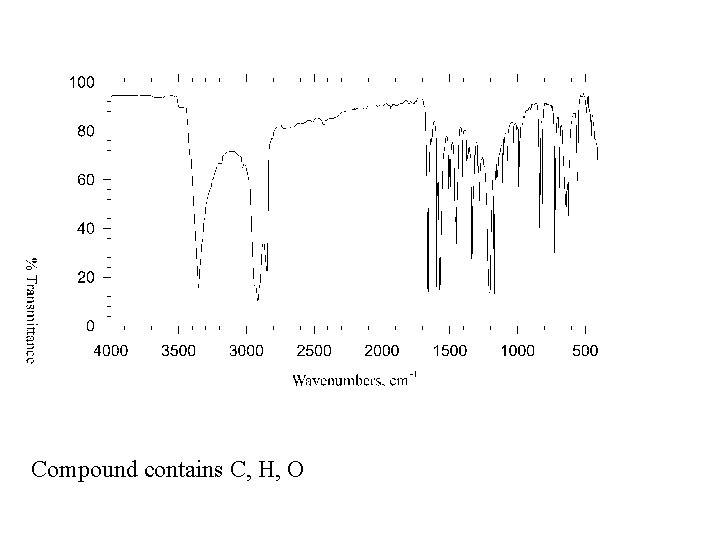
Compound contains C, H, O

 Methyl salicylate classification
Methyl salicylate classification Lewis structure of ch3f
Lewis structure of ch3f 2-methylbutane structure
2-methylbutane structure Myth eth prop
Myth eth prop Methyl
Methyl 17a methyl 1 testosterone
17a methyl 1 testosterone General formula r3n
General formula r3n Nitration of methyl benzoate separation scheme
Nitration of methyl benzoate separation scheme Asetofenon adalah
Asetofenon adalah Methyl ethyl propyl table
Methyl ethyl propyl table Bunsen burger
Bunsen burger Klebsiella
Klebsiella Di-o-methyldynemicin a methyl ester
Di-o-methyldynemicin a methyl ester 1-butanol ir spectrum
1-butanol ir spectrum Calculate the empirical formula of putrescine.
Calculate the empirical formula of putrescine. Discovery of benzene
Discovery of benzene