Rotational Spectroscopy of Methyl Vinyl Ketone OLENA ZAKHARENKO

![Previous studies of MVK Previous study Microwave[1, 2, 3] : 7 – 40 GHz Previous studies of MVK Previous study Microwave[1, 2, 3] : 7 – 40 GHz](https://slidetodoc.com/presentation_image_h2/7266df901a679b27188a303afcab77e0/image-5.jpg)

- Slides: 17

Rotational Spectroscopy of Methyl Vinyl Ketone OLENA ZAKHARENKO, JUAN-RAMON AVILES MORENO, ROMAN A. MOTIYENKO, THERESE R. HUET Laboratoire Ph. LAM, UMR 8523 CNRS - Université Lille 1, Villeneuve d’Ascq, France.

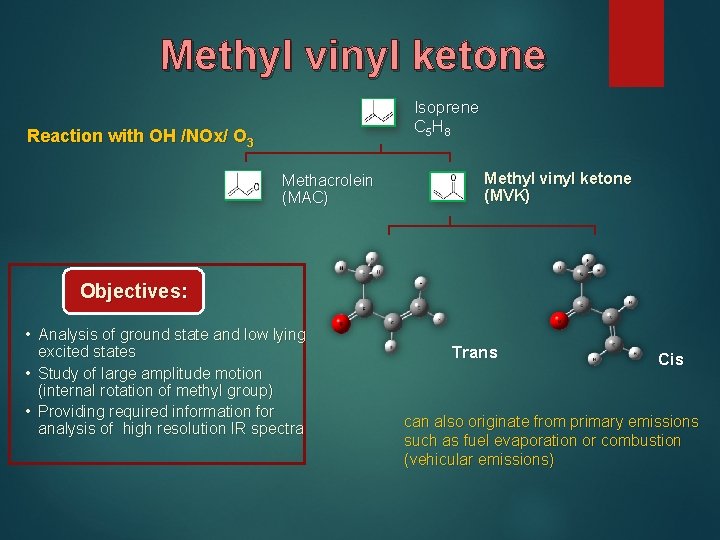
Methyl vinyl ketone Isoprene C 5 H 8 Reaction with OH /NOx/ O 3 Methacrolein (MAC) Methyl vinyl ketone (MVK) Objectives: • Analysis of ground state and low lying excited states • Study of large amplitude motion (internal rotation of methyl group) • Providing required information for analysis of high resolution IR spectra Trans Cis can also originate from primary emissions such as fuel evaporation or combustion (vehicular emissions)

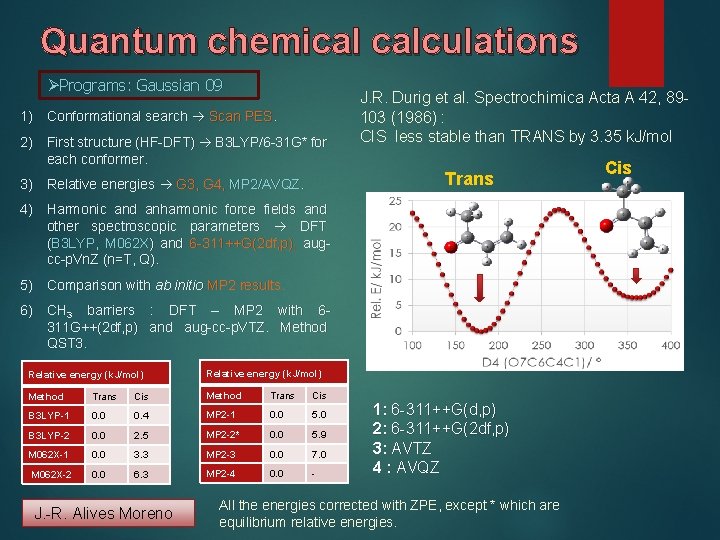
Quantum chemical calculations ØPrograms: Gaussian 09 1) Conformational search Scan PES. 2) First structure (HF-DFT) B 3 LYP/6 -31 G* for each conformer. J. R. Durig et al. Spectrochimica Acta A 42, 89103 (1986) : CIS less stable than TRANS by 3. 35 k. J/mol Trans 3) Relative energies G 3, G 4, MP 2/AVQZ. 4) Harmonic and anharmonic force fields and other spectroscopic parameters DFT (B 3 LYP, M 062 X) and 6 -311++G(2 df, p), augcc-p. Vn. Z (n=T, Q). 5) Comparison with ab initio MP 2 results. 6) CH 3 barriers : DFT – MP 2 with 6311 G++(2 df, p) and aug-cc-p. VTZ. Method QST 3. Relative energy (k. J/mol) Method Trans Cis B 3 LYP-1 0. 0 0. 4 MP 2 -1 0. 0 5. 0 B 3 LYP-2 0. 0 2. 5 MP 2 -2* 0. 0 5. 9 M 062 X-1 0. 0 3. 3 MP 2 -3 0. 0 7. 0 M 062 X-2 0. 0 6. 3 MP 2 -4 0. 0 - J. -R. Alives Moreno 1: 6 -311++G(d, p) 2: 6 -311++G(2 df, p) 3: AVTZ 4 : AVQZ All the energies corrected with ZPE, except * which are equilibrium relative energies. Cis

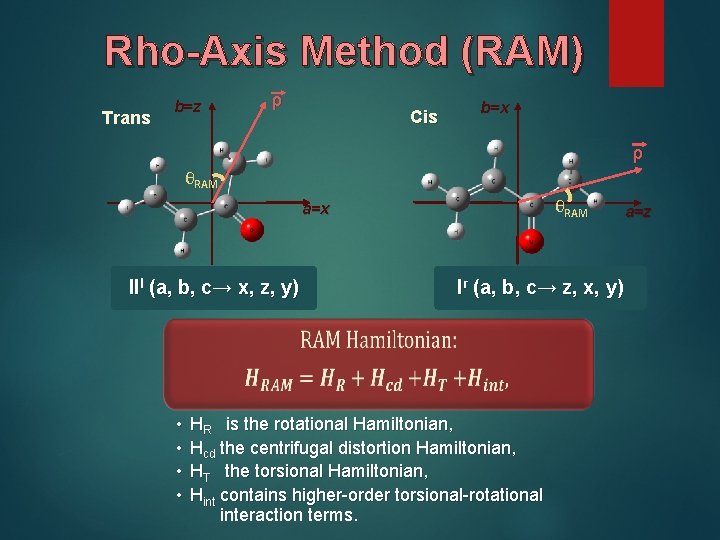
Rho-Axis Method (RAM) Trans b=z ρ Cis b=x ρ θRAM a=x IIl (a, b, c→ x, z, y) • • Ir (a, b, c→ z, x, y) HR is the rotational Hamiltonian, Hcd the centrifugal distortion Hamiltonian, HT the torsional Hamiltonian, Hint contains higher-order torsional-rotational interaction terms. a=z
![Previous studies of MVK Previous study Microwave1 2 3 7 40 GHz Previous studies of MVK Previous study Microwave[1, 2, 3] : 7 – 40 GHz](https://slidetodoc.com/presentation_image_h2/7266df901a679b27188a303afcab77e0/image-5.jpg)
Previous studies of MVK Previous study Microwave[1, 2, 3] : 7 – 40 GHz Trans: up to J = 40 and Ka = 13 Cis: ∙ up to J = 6 and Ka = 4 only ground state Infrared and Raman[4] : 3500 – 10 cm-1 Present study 50 – 650 GHz Trans: up to J = 76 and Ka = 17 Cis: ground state + low lying excited energy states [1] A. C. Fantoni, W. Caminati, R. Meyer, Chem. Phys. Lett. 133 (1987) 27 [2] P. D. Foster, V. M. Rao, R. F. Curl Jr. , J. Chem. Phys. 43 (1965) 1064 [3] D. S. Wilcox, A. J. Shirar, O. L. Williams, B. C. Dian, Chem. Phys. Lett. 508 (2011) 10 -16 [4] J. R. Durig and T. S. Little, J. Chem. Phys. 78 (8), 1981

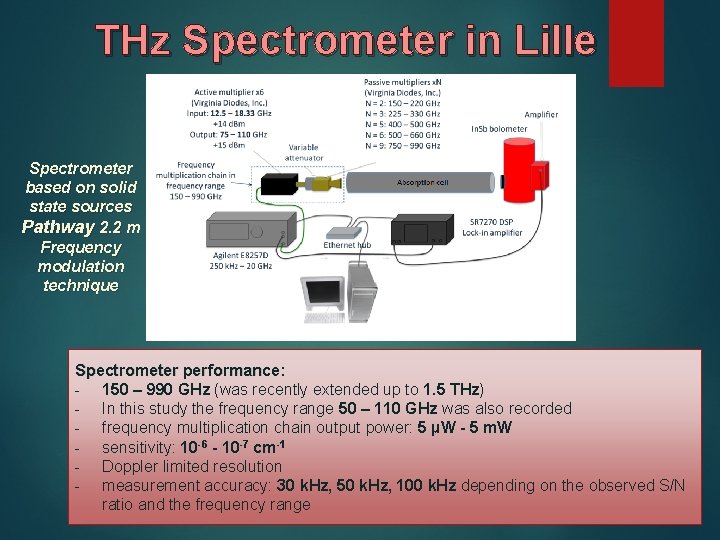
THz Spectrometer in Lille Spectrometer based on solid state sources Pathway 2. 2 m Frequency modulation technique Spectrometer performance: - 150 – 990 GHz (was recently extended up to 1. 5 THz) - In this study the frequency range 50 – 110 GHz was also recorded - frequency multiplication chain output power: 5 µW - 5 m. W - sensitivity: 10 -6 - 10 -7 cm-1 - Doppler limited resolution - measurement accuracy: 30 k. Hz, 50 k. Hz, 100 k. Hz depending on the observed S/N ratio and the frequency range

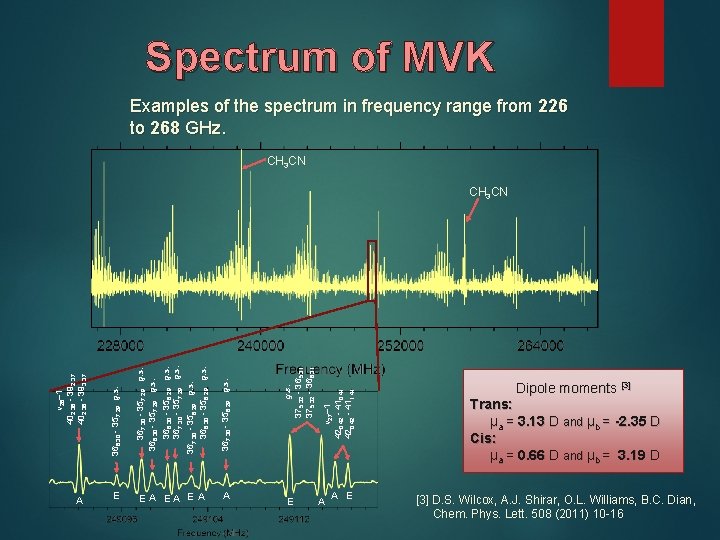
Spectrum of MVK Examples of the spectrum in frequency range from 226 to 268 GHz. CH 3 CN A E E A EA E A A E v 27=1 420, 42 - 410, 41 420, 42 - 411, 41 g. s. 375, 32 - 365, 31 375, 32 - 366, 31 367, 30 - 356, 29 g. s. 366, 30 - 356, 29 g. s. 367, 30 - 357, 29 g. s. 366, 30 - 357, 29 g. s. v 26=1 402, 38 - 392, 37 402, 38 - 393, 37 CH 3 CN A A E Dipole moments [3] Trans: μa = 3. 13 D and μb = -2. 35 D Cis: μa = 0. 66 D and μb = 3. 19 D [3] D. S. Wilcox, A. J. Shirar, O. L. Williams, B. C. Dian, Chem. Phys. Lett. 508 (2011) 10 -16

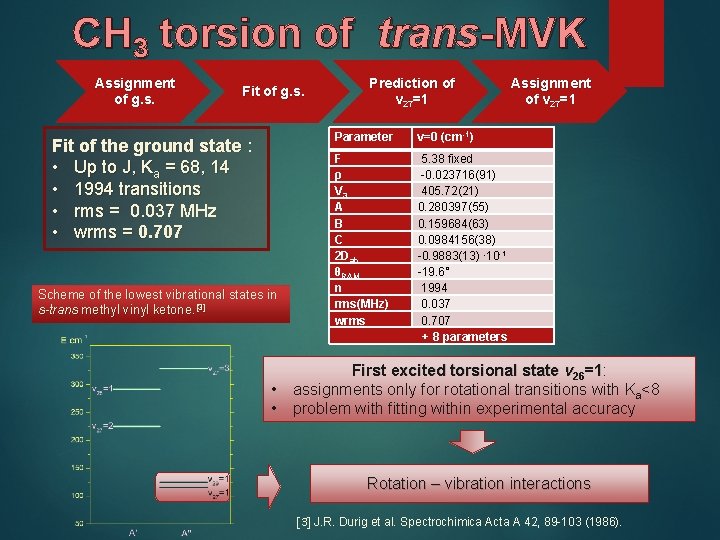
CH 3 torsion of trans-MVK Assignment of g. s. Fit of the ground state : • Up to J, Ka = 68, 14 • 1994 transitions • rms = 0. 037 MHz • wrms = 0. 707 Scheme of the lowest vibrational states in s-trans methyl vinyl ketone. [3] • • Prediction of v 27=1 Parameter v=0 (cm-1) F ρ V 3 A B C 2 Dab θRAM n rms(MHz) wrms 5. 38 fixed -0. 023716(91) 405. 72(21) 0. 280397(55) 0. 159684(63) 0. 0984156(38) -0. 9883(13) ∙ 10 -1 -19. 6° 1994 0. 037 0. 707 + 8 parameters Assignment of v 27=1 First excited torsional state v 26=1: =1 assignments only for rotational transitions with Ka<8 problem with fitting within experimental accuracy Rotation – vibration interactions [3] J. R. Durig et al. Spectrochimica Acta A 42, 89 -103 (1986).

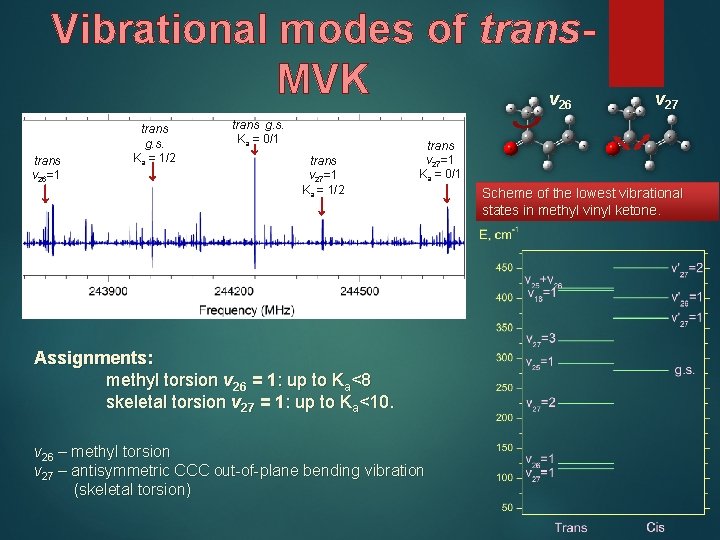
Vibrational modes of trans. MVK v 26 trans v 26=1 trans g. s. Ka = 1/2 trans g. s. Ka = 0/1 trans v 27=1 Ka = 1/2 v 27 trans v 27=1 Ka = 0/1 Assignments: methyl torsion v 26 = 1: up to Ka<8 skeletal torsion v 27 = 1: up to Ka<10. v 26 – methyl torsion v 27 – antisymmetric CCC out-of-plane bending vibration (skeletal torsion) Scheme of the lowest vibrational states in methyl vinyl ketone.

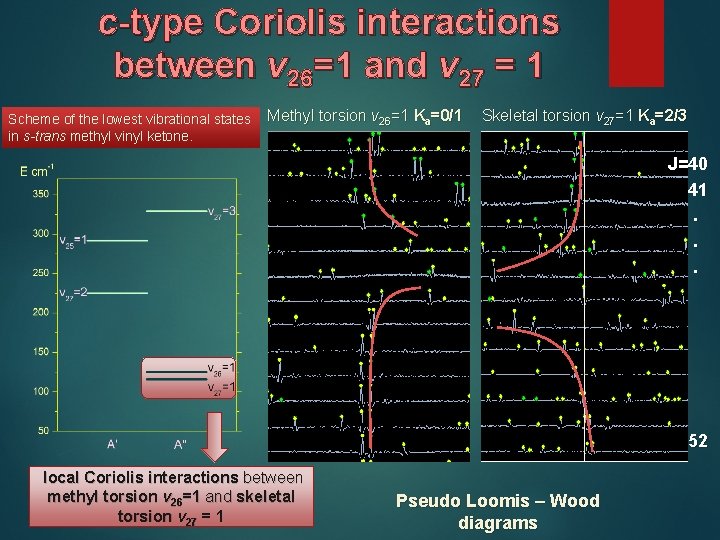
c-type Coriolis interactions between v 26=1 and v 27 = 1 Scheme of the lowest vibrational states in s-trans methyl vinyl ketone. Methyl torsion v 26=1 Ka=0/1 Skeletal torsion v 27=1 Ka=2/3 J=40 41. . . 52 local Coriolis interactions between methyl torsion v 26=1 and skeletal torsion v 27 = 1 Pseudo Loomis – Wood diagrams

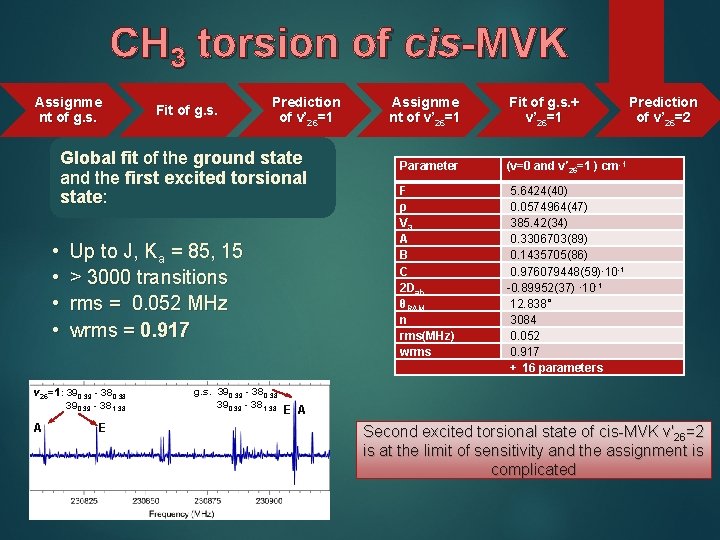
CH 3 torsion of cis-MVK Assignme nt of g. s. Fit of g. s. Prediction of v’ 26=1 Global fit of the ground state and the first excited torsional state: • • Up to J, Ka = 85, 15 > 3000 transitions rms = 0. 052 MHz wrms = 0. 917 v 26=1: 390, 39 - 380, 38 390, 39 - 381, 38 A E g. s. 390, 39 - 380, 38 390, 39 - 381, 38 Assignme nt of v’ 26=1 Fit of g. s. + v’ 26=1 Parameter (v=0 and v′ 26=1 ) cm-1 F ρ V 3 A B C 2 Dab θRAM n rms(MHz) wrms 5. 6424(40) 0. 0574964(47) 385. 42(34) 0. 3306703(89) 0. 1435705(86) 0. 976079448(59)∙ 10 -1 -0. 89952(37) ∙ 10 -1 12. 838° 3084 0. 052 0. 917 + 16 parameters Prediction of v’ 26=2 E A Second excited torsional state of cis-MVK v′ 26=2 is at the limit of sensitivity and the assignment is complicated

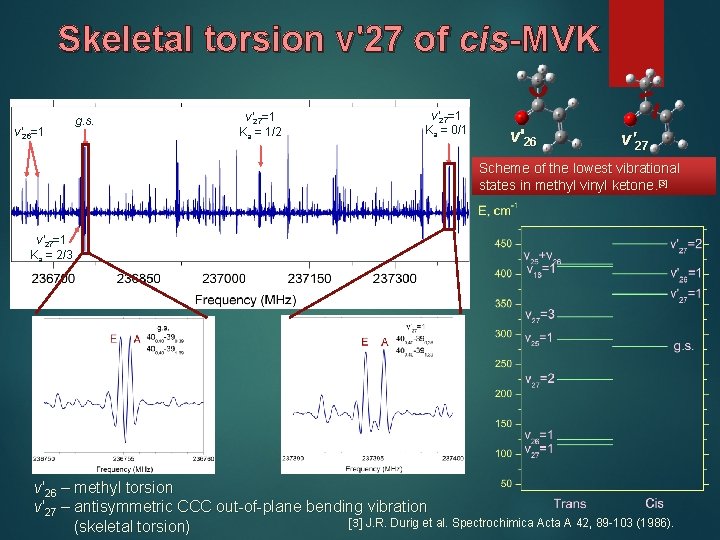
Skeletal torsion v'27 of cis-MVK v′ 26=1 g. s. v′ 27=1 Ka = 1/2 v′ 27=1 Ka = 0/1 v'26 v'27 Scheme of the lowest vibrational [3] states in methyl vinyl ketone. [3] v′ 27=1 Ka = 2/3 v'26 – methyl torsion v'27 – antisymmetric CCC out-of-plane bending vibration [3] J. R. Durig et al. Spectrochimica Acta A 42, 89 -103 (1986). (skeletal torsion)

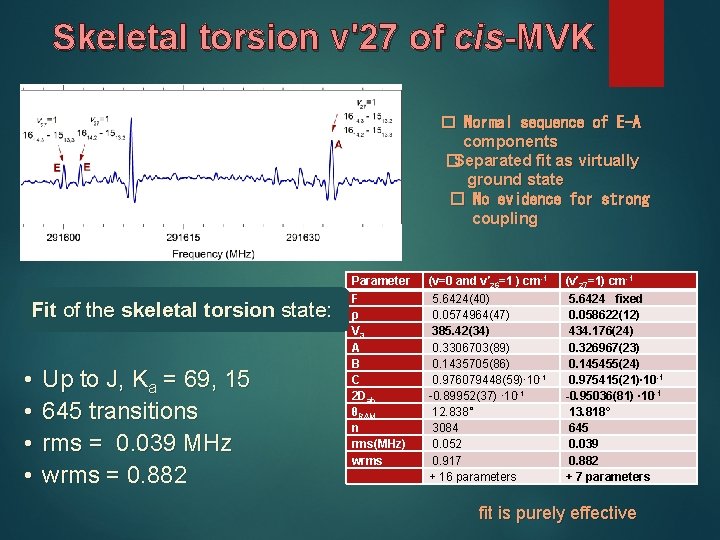
Skeletal torsion v'27 of cis-MVK � Normal sequence of E-A components �Separated fit as virtually ground state � No evidence for strong coupling Fit of the skeletal torsion state: • • Up to J, Ka = 69, 15 645 transitions rms = 0. 039 MHz wrms = 0. 882 Parameter (v=0 and v′ 26=1 ) cm-1 (v′ 27=1) cm-1 F ρ V 3 A B C 2 Dab θRAM n rms(MHz) wrms 5. 6424(40) 0. 0574964(47) 385. 42(34) 0. 3306703(89) 0. 1435705(86) 0. 976079448(59)∙ 10 -1 -0. 89952(37) ∙ 10 -1 12. 838° 3084 0. 052 0. 917 + 16 parameters 5. 6424 fixed 0. 058622(12) 434. 176(24) 0. 326967(23) 0. 145455(24) 0. 975415(21)∙ 10 -1 -0. 95036(81) ∙ 10 -1 13. 818° 645 0. 039 0. 882 + 7 parameters fit is purely effective

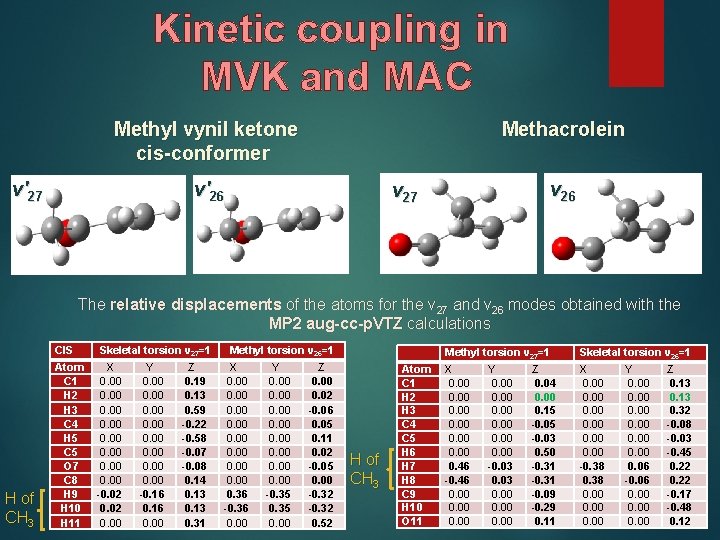
Kinetic coupling in MVK and MAC Methyl vynil ketone cis-conformer Methacrolein v′ 26 v′ 27 v 26 v 27 The relative displacements of the atoms for the ν 27 and ν 26 modes obtained with the MP 2 aug-cc-p. VTZ calculations H of CH 3 CIS Skeletal torsion v 27=1 Atom C 1 H 2 H 3 C 4 H 5 C 5 O 7 C 8 H 9 H 10 H 11 X 0. 00 0. 00 -0. 02 0. 00 Y 0. 00 0. 00 -0. 16 0. 00 Z 0. 19 0. 13 0. 59 -0. 22 -0. 58 -0. 07 -0. 08 0. 14 0. 13 0. 31 Methyl torsion v 26=1 X 0. 00 0. 36 -0. 36 0. 00 Y 0. 00 0. 00 -0. 35 0. 00 Z 0. 00 0. 02 -0. 06 0. 05 0. 11 0. 02 -0. 05 0. 00 -0. 32 0. 52 H of CH 3 Atom C 1 H 2 H 3 C 4 C 5 H 6 H 7 H 8 C 9 H 10 O 11 Methyl torsion v 27=1 Skeletal torsion v 26=1 X 0. 00 0. 46 -0. 46 0. 00 X 0. 00 -0. 38 0. 00 Y 0. 00 -0. 03 0. 00 Z 0. 04 0. 00 0. 15 -0. 03 0. 50 -0. 31 -0. 09 -0. 29 0. 11 Y 0. 00 0. 06 -0. 06 0. 00 Z 0. 13 0. 32 -0. 08 -0. 03 -0. 45 0. 22 -0. 17 -0. 48 0. 12

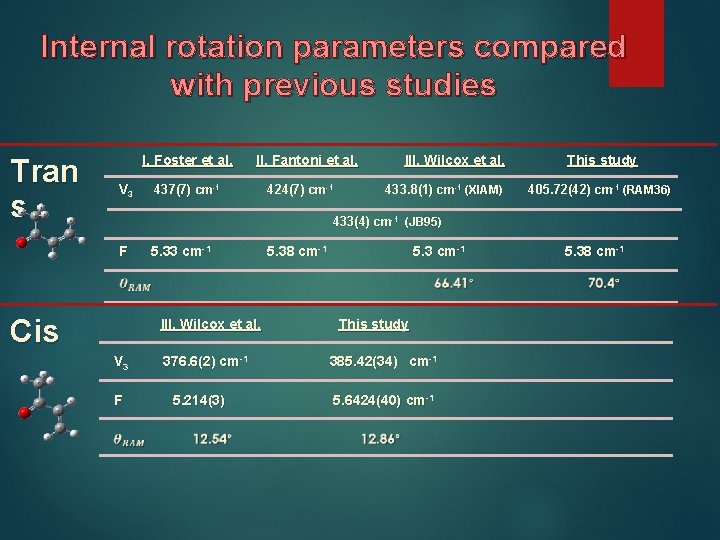
Internal rotation parameters compared with previous studies Tran s I. Foster et al. V 3 II. Fantoni et al. 437(7) cm-1 424(7) cm-1 III. Wilcox et al. 433. 8(1) cm-1 (XIAM) This study 405. 72(42) cm-1 (RAM 36) 433(4) cm-1 (JB 95) F Cis 5. 33 cm-1 III. Wilcox et al. V 3 F 376. 6(2) cm-1 5. 214(3) 5. 38 cm-1 5. 3 cm-1 This study 385. 42(34) cm-1 5. 6424(40) cm-1 5. 38 cm-1

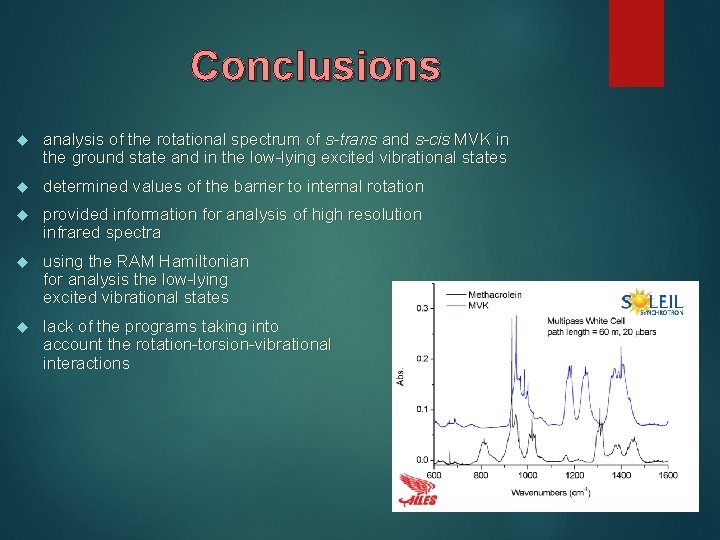
Conclusions analysis of the rotational spectrum of s-trans and s-cis MVK in the ground state and in the low-lying excited vibrational states determined values of the barrier to internal rotation provided information for analysis of high resolution infrared spectra using the RAM Hamiltonian for analysis the low-lying excited vibrational states lack of the programs taking into account the rotation-torsion-vibrational interactions

THANK YOU FOR YOUR ATTENTION Support from the Ca. PPA project (Chemical and Physical Properties of the Atmosphere) is acknowledged. Ca. PPA is funded by the French National Research Agency (ANR) through the PIA (Programme d'Investissement d'Avenir) under contract "ANR-11 -LABX-0005 -01" and by the Regional Council “ Nord-Pas de Calais » and the "European Funds for Regional Economic Development (FEDER)
 Methyl vinyl silicone gum
Methyl vinyl silicone gum Olena maslyukivska
Olena maslyukivska Olena kononenko
Olena kononenko Hyperphysics
Hyperphysics Gross selection rule for rotational spectroscopy
Gross selection rule for rotational spectroscopy Rotational spectroscopy notes
Rotational spectroscopy notes Application of rotational spectroscopy
Application of rotational spectroscopy Microwave spectroscopy
Microwave spectroscopy Rotational equilibrium and dynamics
Rotational equilibrium and dynamics Second condition of equilibrium
Second condition of equilibrium Polymer vinyl
Polymer vinyl Scotchgard vinyl protector
Scotchgard vinyl protector Alkyl aryl
Alkyl aryl Notifier id3000 software download
Notifier id3000 software download Cationic vinyl polymerization
Cationic vinyl polymerization Nucleophilic substitution mechanism
Nucleophilic substitution mechanism Vinyl radical
Vinyl radical Vinyl vegyipari kft
Vinyl vegyipari kft