Menthol Dr Javid Hussain 1 Chemical Properties S




- Slides: 20

Menthol Dr. Javid Hussain 1

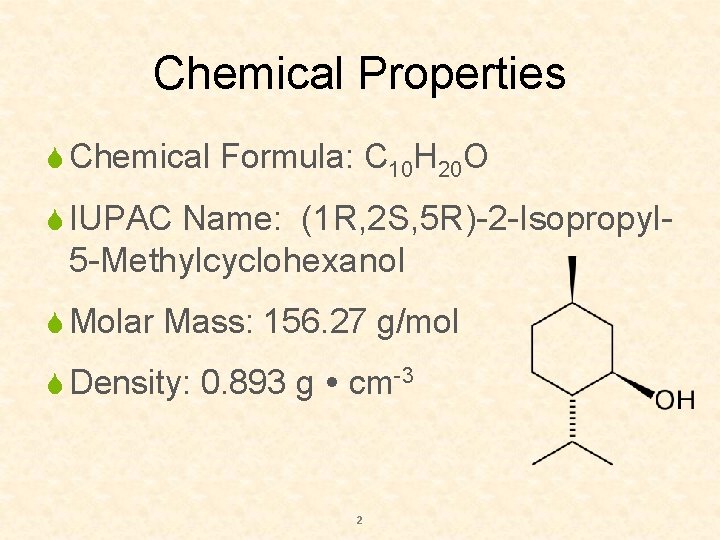
Chemical Properties S Chemical Formula: C 10 H 20 O S IUPAC Name: (1 R, 2 S, 5 R)-2 -Isopropyl- 5 -Methylcyclohexanol S Molar Mass: 156. 27 g/mol S Density: 0. 893 g cm-3 2

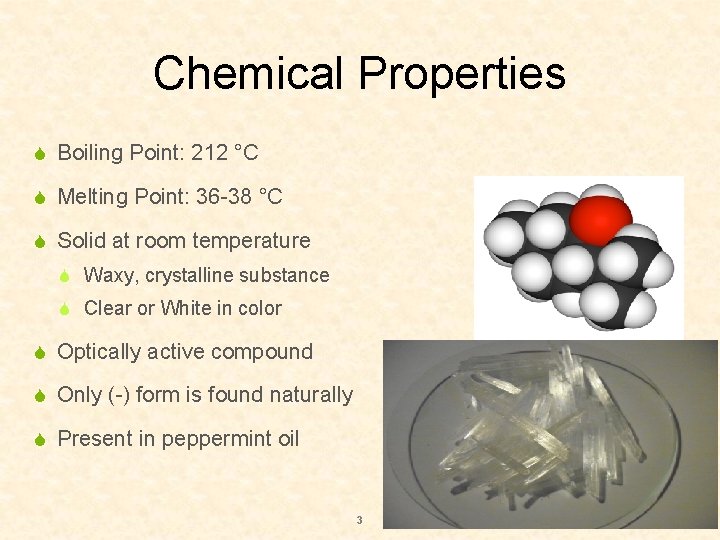
Chemical Properties S Boiling Point: 212 °C S Melting Point: 36 -38 °C S Solid at room temperature S Waxy, crystalline substance S Clear or White in color S Optically active compound S Only (-) form is found naturally S Present in peppermint oil 3

Medicinal Uses 4

Products Found In 5

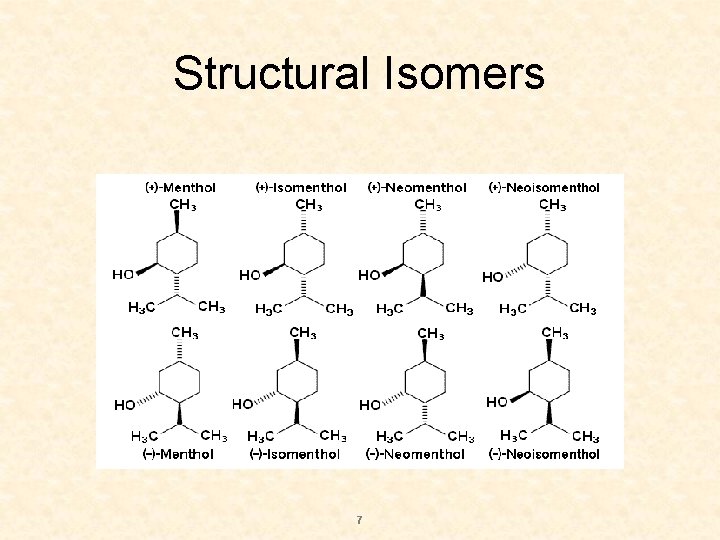
Structural Isomers 7

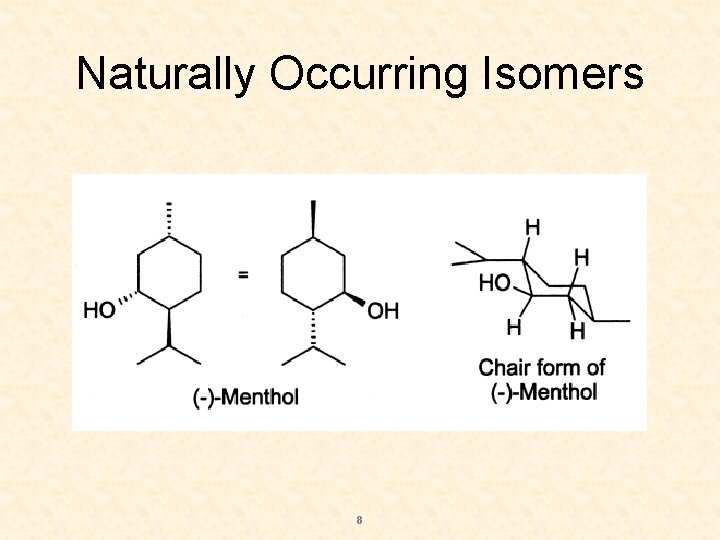
Naturally Occurring Isomers 8

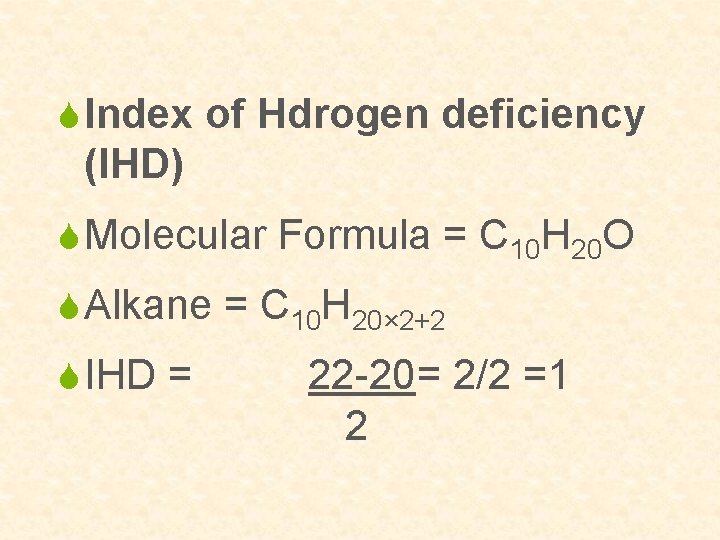
S Index of Hdrogen deficiency (IHD) S Molecular Formula = C 10 H 20 O S Alkane = C 10 H 20× 2+2 S IHD = 22 -20= 2/2 =1 2

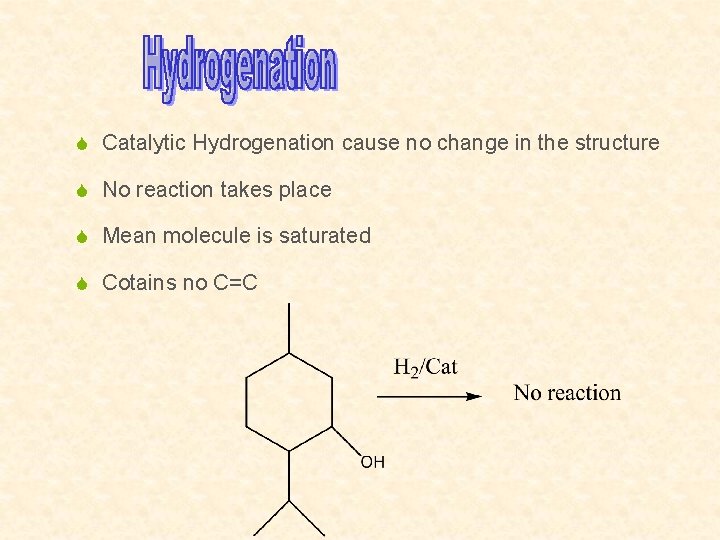
S Catalytic Hydrogenation cause no change in the structure S No reaction takes place S Mean molecule is saturated S Cotains no C=C

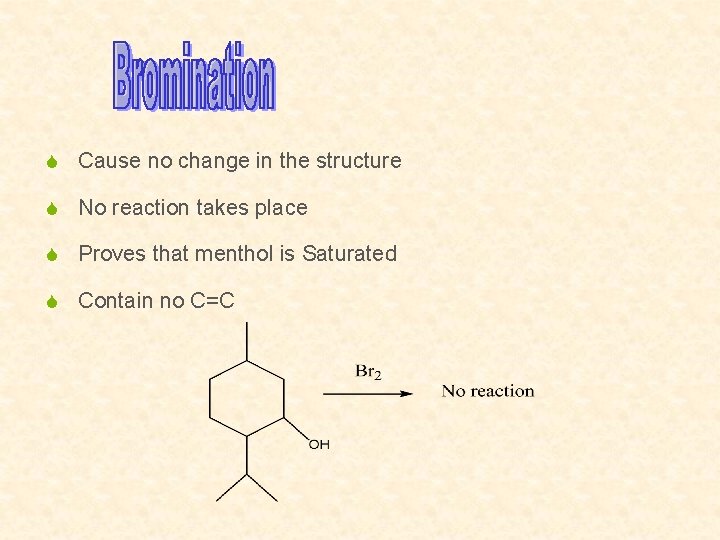
S Cause no change in the structure S No reaction takes place S Proves that menthol is Saturated S Contain no C=C

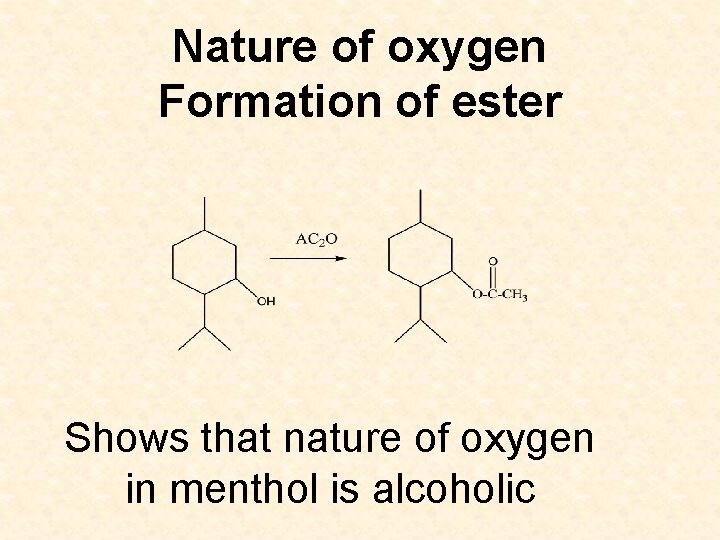
Nature of oxygen Formation of ester Shows that nature of oxygen in menthol is alcoholic

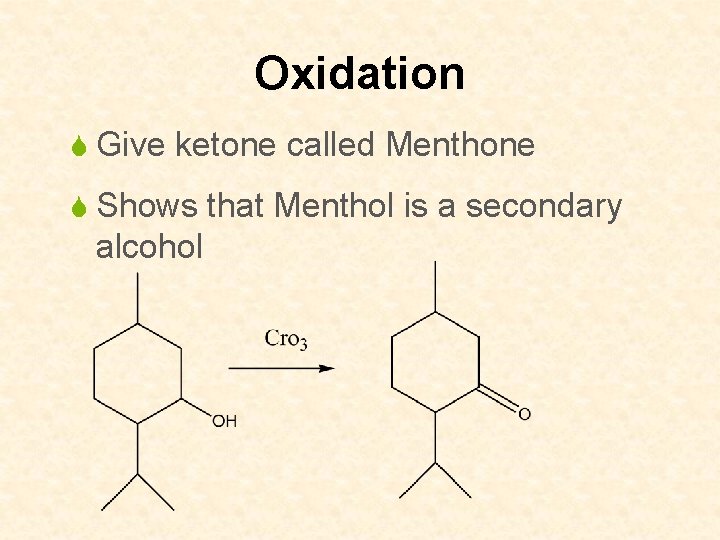
Oxidation S Give ketone called Menthone S Shows that Menthol is a secondary alcohol

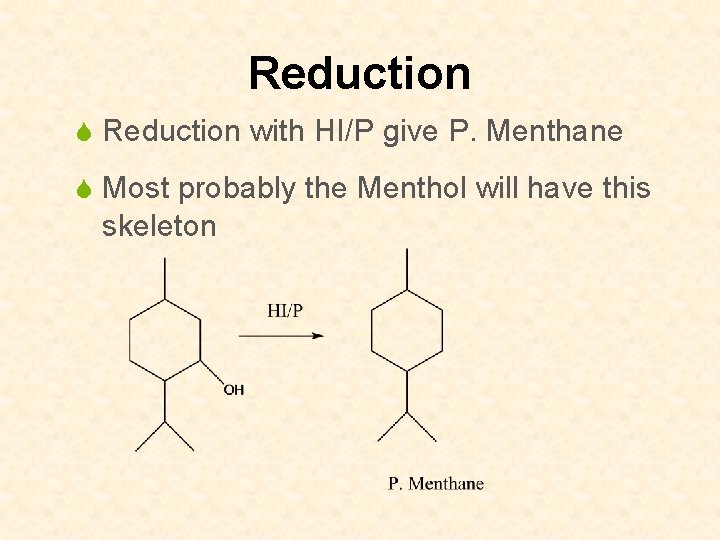
Reduction S Reduction with HI/P give P. Menthane S Most probably the Menthol will have this skeleton

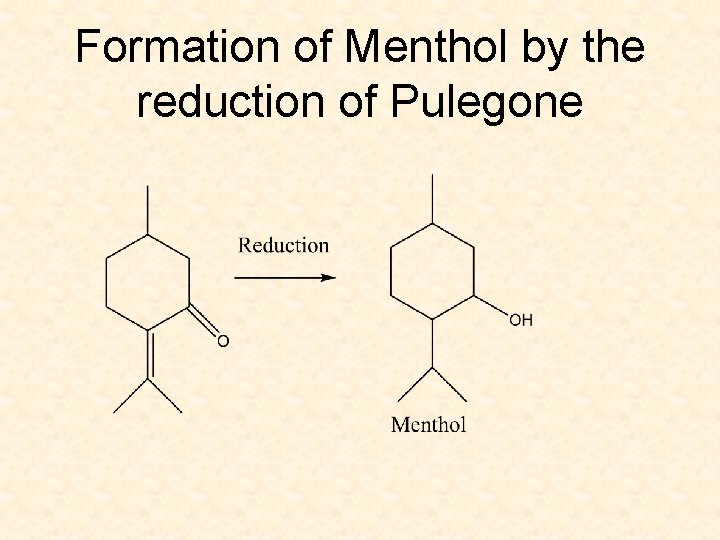
Formation of Menthol by the reduction of Pulegone

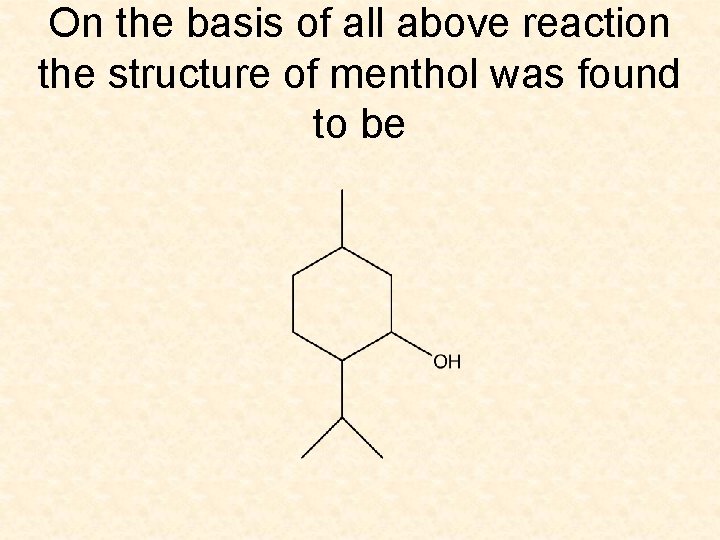
On the basis of all above reaction the structure of menthol was found to be

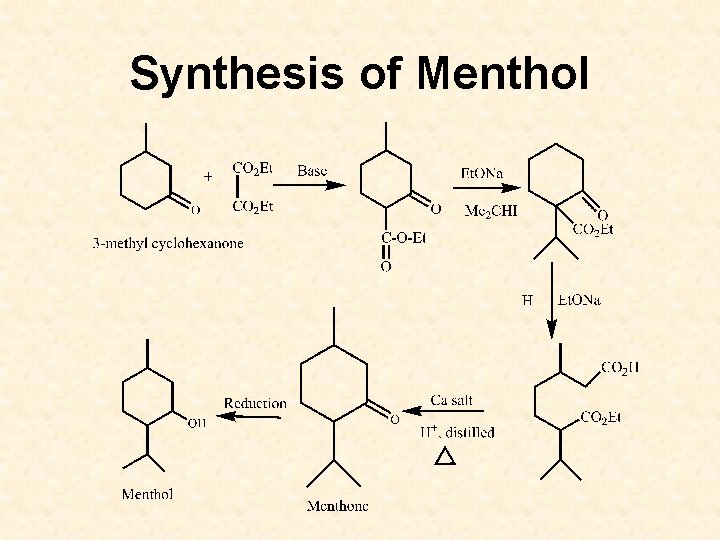
Synthesis of Menthol

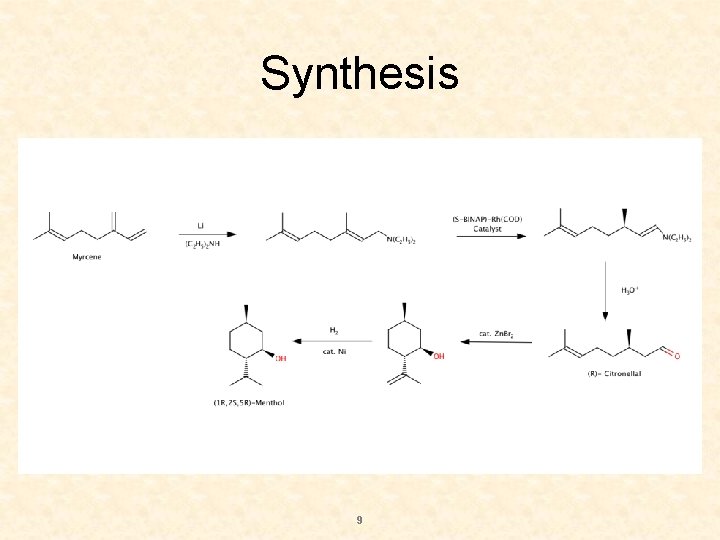
Synthesis 9

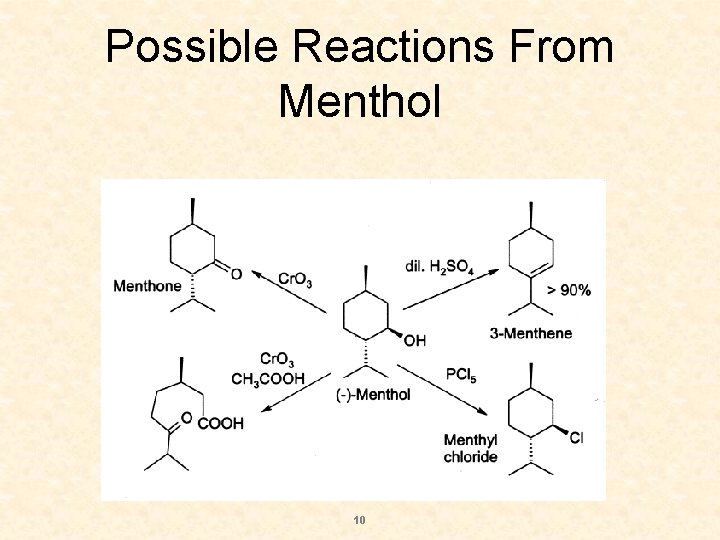
Possible Reactions From Menthol 10

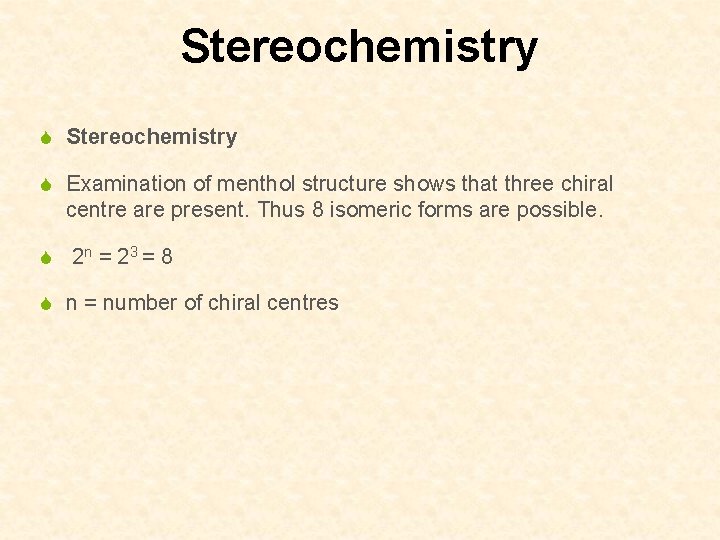
Stereochemistry S Examination of menthol structure shows that three chiral centre are present. Thus 8 isomeric forms are possible. S 2 n = 2 3 = 8 S n = number of chiral centres

Acknowledgments 12
 Javid rzayev
Javid rzayev Javid abdullayev
Javid abdullayev Javid dadashkarimi
Javid dadashkarimi In the mixing of thymol and menthol
In the mixing of thymol and menthol Schudmengsel
Schudmengsel Why is adding menthol to cigarettes dangerous?
Why is adding menthol to cigarettes dangerous? Basis polietilenglikol inkompatibel dengan bahan obat ini
Basis polietilenglikol inkompatibel dengan bahan obat ini Is smell a physical property
Is smell a physical property Ishaat hussain tata
Ishaat hussain tata Imam hussain family tree
Imam hussain family tree Wpi stimulator
Wpi stimulator Waris husain
Waris husain Sehrish hussain
Sehrish hussain Iftikhar hussain md
Iftikhar hussain md Shahid hussain psychiatrist
Shahid hussain psychiatrist Hussain mujhko maaf karna lyrics
Hussain mujhko maaf karna lyrics Hussain
Hussain Zahin hussain
Zahin hussain Mulazim hussain bukhari
Mulazim hussain bukhari Hussain manawer poems
Hussain manawer poems Kabeer hussain
Kabeer hussain